CASE PRESENTATION
A 75-year-old man is referred for a cataract surgery evaluation. The patient has experienced a progressive, painless decrease in vision over the past year. He reports that his near vision is not correctable, and he struggles to read menus and fine print. He has difficulty driving at night owing to glare from headlights and trouble reading street signs.
The patient wears progressive spectacles to correct moderate myopia. He rarely takes his glasses off for near work except for specific tasks such as removing a splinter. The patient’s uncorrected distance visual acuity is 20/200 OU. His uncorrected near visual acuity is J3 OD, J2 near OS, and J2 OU. His BSCVA is 20/60 and J5 OD and 20/40 and J1 OS. His binocular BSCVA is 20/40+2 and J1. On examination, the patient’s BCVA is 20/25- and J5 OD with a manifest refraction of -2.50 -0.75 x 133º and an add of +2.00 D. His BCVA is 20/30- and J1 OS with a manifest refraction of -2.00 -0.50 x 110º and an add of +2.00 D.
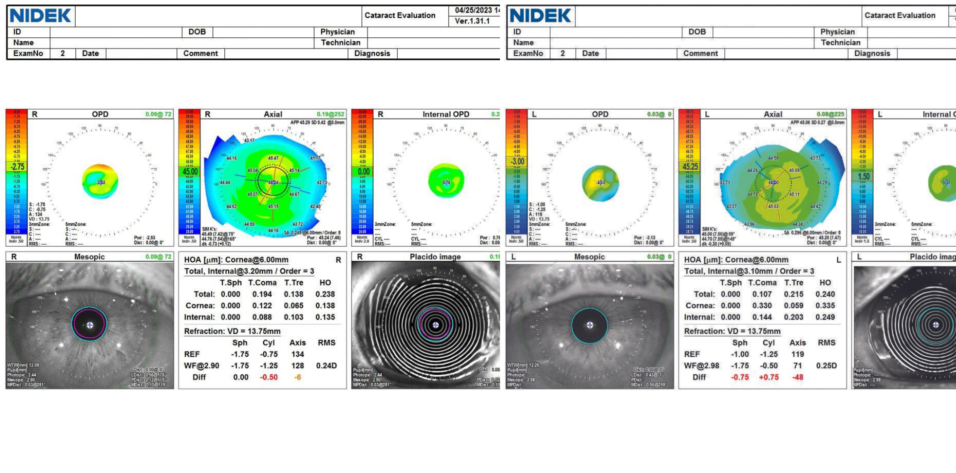
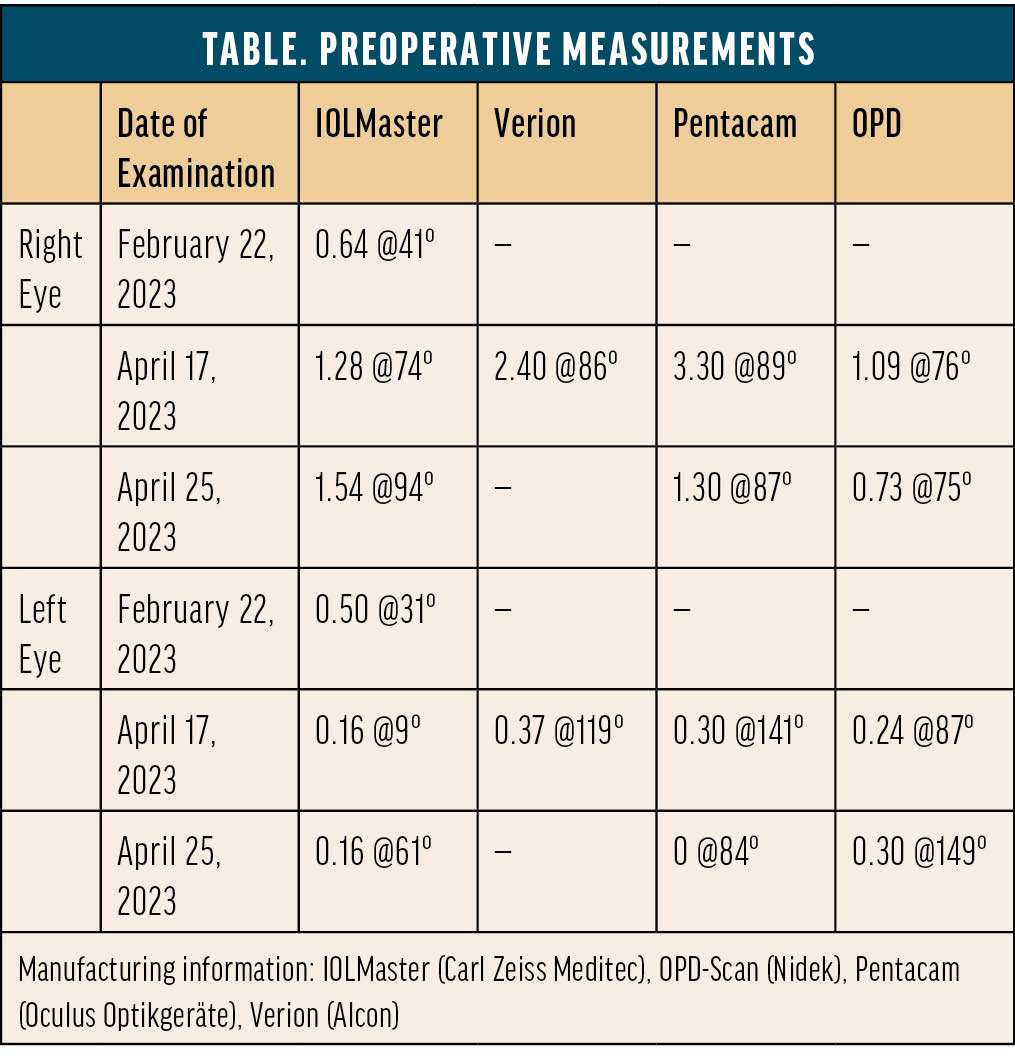
The patient’s medical history is notable for hypertension, benign prostatic hyperplasia for which he is administering tamsulosin, and hypercholesterolemia. He has no history of surgery or ocular conditions other than myopia. An ocular examination reveals cataracts and mild meibomian gland dysfunction. OCT imaging of the macula is normal. The Table shows the results of aberrometry and topography.
The patient has never worn contact lenses and thus has no experience with monovision or multifocality. He has an easy-going personality, is an administrator at his church, and enjoys going for walks and gardening in his free time. The patient would like to avoid wearing glasses as much as possible after cataract surgery. His main intermediate and near tasks are some computer and desk work and looking at his phone. Most of his driving is over short distances during the day, but nighttime driving is somewhat important to him.
After a thorough discussion of IOL options, the patient expresses great interest in a trifocal lens for both eyes. Given that the vision in his dominant right eye is significantly impaired by a cataract, surgery is planned for that eye first.
It is thoroughly and repeatedly explained to the patient that his near vision with any IOL that corrects distance vision will be less crisp than his natural uncorrected near visual acuity as a person with myopia. The visual side effects of multifocality and the importance of treating ocular surface disease (OSD) to increase the reliability of preoperative measurements, imaging, and IOL calculations and postoperative visual outcomes, comfort, and recovery are also discussed. OSD management is initiated, and ocular imaging is repeated (Table). The axis and magnitude of astigmatism are extremely variable across days and devices. OSD treatment is escalated to include a topical immunomodulator. The patient returns for a third set of measurements, which are again variable and inconsistent (Table, Figures 1 and 2).
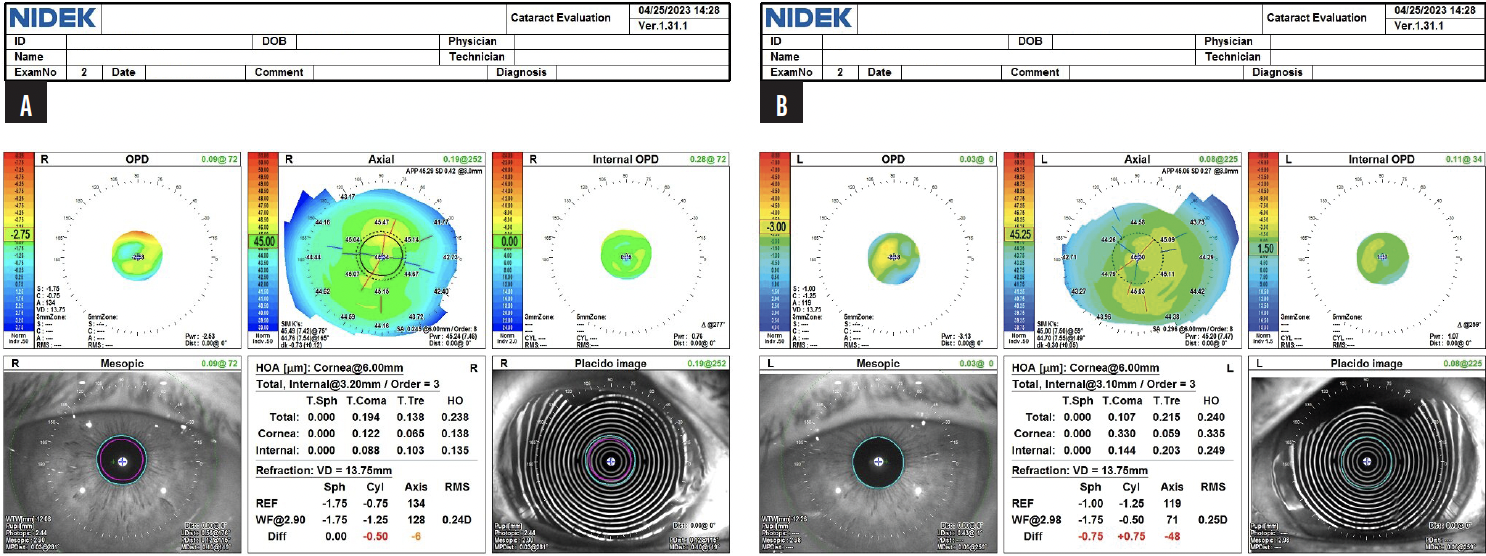
Figure 1. OPD scans for the right (A) and left (B) eyes from the final visit before cataract surgery on the first eye.
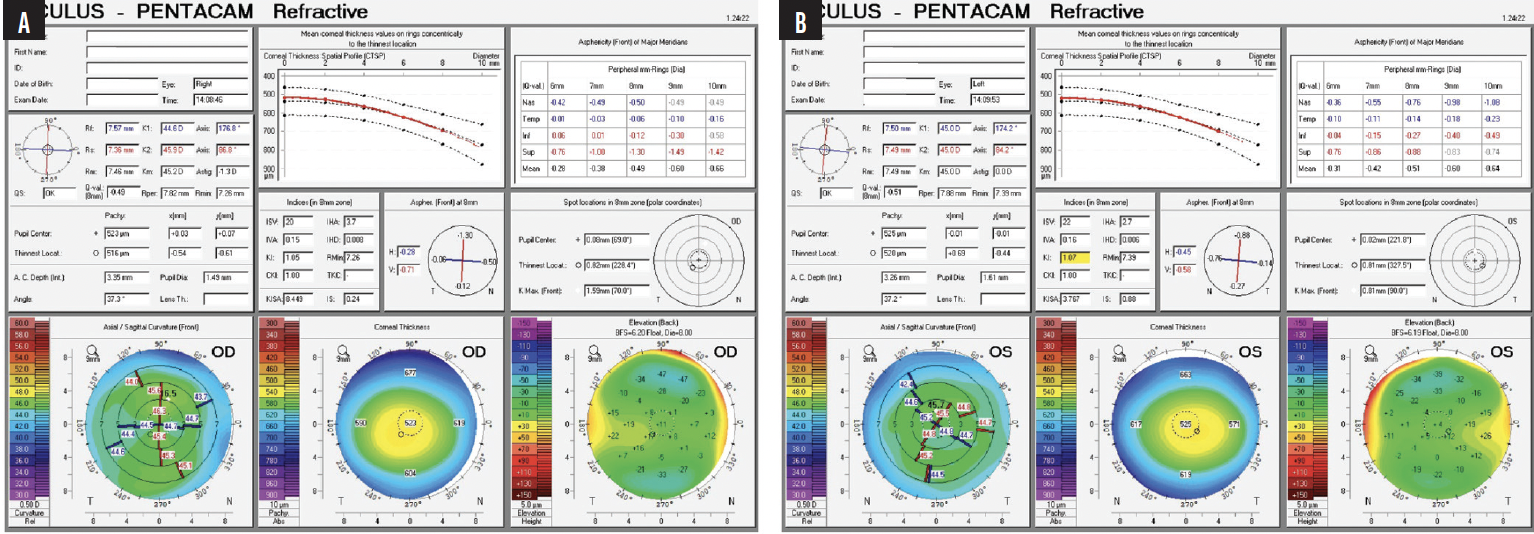
Figure 2. Pentacam scans for the right (A) and left (B) eyes from the final visit before cataract surgery on the first eye.
The patient reports adhering to prescribed OSD treatment, which includes warm compresses, lid scrubs, artificial tears, topical immunomodulator, and topical loteprednol eye drops. An examination shows a relatively stable tear film and a tear breakup time of 8 to 10 seconds in each eye. Surgery is scheduled for next week, and he does not wish to delay the procedure.
How many times would you repeat measurements, and what treatments would you add or modify to maximize improvement in preoperative measurements and imaging in the shortest amount of time? Have you found that, for some patients, measurements continue to be variable no matter what treatment is initiated and how long surgery is deferred? What do you recommend in that situation?
In the current case, would you proceed with cataract surgery and trifocal IOL implantation if the patient wishes? If so, how would you educate him about his possible future need for an enhancement? If not, which IOL would you recommend, and how would you counsel the patient?
— Case prepared by Neda Nikpoor, MD

ARJAN HURA, MD
The patient is interested in spectacle independence through the implantation of a multifocal IOL but has inconsistent preoperative measurements, likely secondary to OSD despite multimodal treatment. He does not wish to delay surgery any longer.
In situations like this one, it may be best to inform patients that they are not good candidates for a multifocal IOL. Quality of vision is directly affected by the ocular surface and air-tear film interface. Variable measurements make hitting the desired postoperative refractive target difficult. The diffractive optics of multifocal IOLs tend not to provide a high quality of vision in the setting of OSD.
The current patient has a stable tear film and decent tear breakup time. Assuming the examination is otherwise normal, the variable preoperative measurements suggest that the ocular surface is still not optimal. Is he willing to use warm compresses, artificial tears, a topical immunomodulator, and topical loteprednol indefinitely after surgery? If not, the health of the ocular surface is likely to regress somewhat and his quality of vision with it.
The possible future need for a laser vision correction (LVC) enhancement poses a similar quandary. Should LVC be performed without a stable refraction? Should it be performed in the setting of significant OSD? Would it be appropriate to perform hyperopic LVC on a pseudophakic eye that has diffractive optics?
I would inform the patient that he is not a good candidate for a multifocal IOL in the right eye and would recommend a monofocal IOL instead. The level of cylinder in the left eye is low (< 0.50 D), so it would be reasonable to have a nuanced conversation with the patient about the pros and cons of implanting a multifocal or extended depth of focus (EDOF) IOL. There is no guarantee of a successful outcome, however, with either premium IOL design, which must be stressed to the patient. Intraoperative aberrometry could help determine which IOL power to implant in each eye.

BRETT MUELLER, DO, PHD
My approach to situations like this one is to optimize the ocular surface. The initial course is typically artificial tears, a topical immunomodulator, and a topical low-dose steroid. Patients return every 2 weeks for an evaluation of the ocular surface and the stability of corneal measurements.
I would feel comfortable implanting a trifocal IOL in the patient’s left eye. Another set of corneal measurements for the right eye would be obtained. If the magnitude and axis of astigmatism are the same in 2 weeks, I would feel comfortable placing a trifocal IOL in the right eye as well. The patient would be counseled that OSD treatment will likely be required after cataract surgery for him to achieve a high quality of vision with a trifocal IOL.
Alternatively, a trifocal and a monofocal toric IOL could be implanted in the left and right eyes, respectively. I give myopic patients the option of a distance or near target (-1.75 to -2.00 D) with a monofocal toric IOL. I gravitate toward this lens option in patients who I think will continue to experience dry eye disease (DED) after cataract surgery.
A third option would be to place a nondiffractive EDOF IOL or a Light Adjustable Lens (LAL; RxSight) in the right eye.
I would explain to the patient that monofocal toric and nondiffractive EDOF IOLs are more forgiving than trifocal IOLs when OSD is present.

BRIAN SHAFER, MD
Carpenters recommend measuring twice and cutting once. I think refractive cataract surgeons should measure thrice and cut once. Unfortunately, this can backfire, as illustrated by the case presentation. The measurements obtained on three separate dates are completely different, especially in the right eye.
I would not feel comfortable implanting a trifocal IOL in the right eye. An appreciable but fluctuating amount of astigmatism would likely benefit from correction, but it is impossible to determine how much to address or where. Given the patient’s desire to proceed with surgery, I would buy time by operating on the left eye first. It has minimal astigmatism that does not warrant correction. A trifocal IOL would be implanted.
Therapy with a topical steroid such as loteprednol would be initiated. The drug would be administered four times daily for 1 week, then twice daily for 1 week. At the first postoperative visit for the left eye, measurements of the right eye would be repeated. It is to be hoped that they are consistent and clean. If they are not, the patient would be informed that he is not a candidate for a trifocal IOL in this eye.
Depending on pupillary dilation and given the patient’s treatment with tamsulosin, an LAL would be a reasonable choice for the dominant right eye. If the measurements of astigmatism become more consistent between devices, a toric monofocal IOL targeted for distance could also be considered to optimize his distance vision. Either way, it would be critical to achieve consistent measurements before proceeding to surgery.

DAGNY ZHU, MD
The patient’s desired outcome is not realistically attainable. High-quality postoperative vision and spectacle independence depend not only on the IOL implanted but also the patient’s inherent optical system. The latter is usually more important.
A suboptimal ocular surface greatly hinders the performance of a trifocal IOL. The variability in biometry and topography measurements suggests DED. If tear osmolarity testing identifies the presence of evaporative DED, in-office expression of the meibomian glands could be performed. In my experience, these patients require long-term treatment and do not show significant improvement in time for cataract surgery. I would therefore avoid a multifocal IOL and instead recommend a nondiffractive EDOF IOL, which would be less sensitive to OSD and could increase the patient’s spectacle independence.
An LAL would be another option. Customization of the refractive outcome postoperatively allowed similar patients of mine to achieve a high level of spectacle independence with a mini-monovision approach (≤ -1.00 D in the nondominant eye). I find that even individuals who have not tried monovision previously can find success with blended vision.

WHAT I DID: NEDA NIKPOOR, MD
As the panelists discuss, in cases like this one, it is important to optimize the ocular surface and repeat measurements to ensure a good refractive outcome. Sometimes, however, OSD measurements are so variable that the process drags on and frustrates the patient. This is particularly true when the person is not experiencing symptoms from their DED but is irritated by their blurry vision due to cataracts.
In the case presented, despite aggressive treatment of the ocular surface, the amount of astigmatism continued to vary across multiple sets of measurements obtained over 3 days. At that point, the patient and I discussed the option of an LAL. I explained that implantation of this lens type would allow surgery to occur as planned and treatment of the ocular surface to continue before refractive adjustments (ie, light treatments) were made.
Four weeks after surgery on the second eye, the patient’s refraction was stable at -0.25 -0.50 x 98º OD and +1.25 -0.75 x 164º OS. One treatment each was performed on the right and left eyes with targets of -0.75 D and plano, respectively. One week later, his UCVA was 20/15 and J5 OS, 20/40-1 and J1+ OD, and 20/15 and J1+ OU. The patient’s refraction was -0.75 -0.25 x 83º OD and +0.25 -0.50 x 100º OS. The patient was thrilled with his vision, so lock-in treatments were performed. Six weeks afterward, his refraction, UCVA, and level of satisfaction were unchanged.
Not all my patients with an unstable tear film have achieved similar success after just 1 additional month of treatment or required only one light treatment. The current case nevertheless shows that the LAL can be an excellent option for individuals with variable topographic and biometric astigmatism. Instead of the surgeon’s trying to identify and treat the correct axis with diagnostic devices, the patient’s postoperative refraction guides treatment until they are happy with their visual acuity.
It remains important to optimize the ocular surface. Refractive instability or fluctuating vision prompts postponement of a light adjustment, an escalation of ocular surface treatment, and repetition of the refraction 1 week later. Only when a patient’s refraction is stable is a light adjustment performed.
When an eye has a low amount of astigmatism (< 1.50 D), measurements across devices and days may vary greatly. This can make choosing the magnitude and axis of alignment for a toric IOL challenging. Results, moreover, may be unpredictable. I find the chances of a successful outcome to be greater when an LAL is implanted in these eyes. It remains important, however, to attempt to stabilize the tear film, which can affect the patient’s refraction and thus the light adjustments and the individual’s final visual acuity.