The world is slowly coming to terms with the fallout from the COVID-19 pandemic more than 2 years after it began. When SARS-CoV-2 first struck, little was known about the virus or the illness it causes. Many of the strategies for its prevention and treatment assumed that SARS-CoV-2 would behave similarly to other viruses. It quickly became apparent that SARS-CoV-2 is alarmingly different and that many management strategies were ineffective. Collaborative scientific research from around the world has increased our understanding of the virus and COVID-19 and led to the development of effective vaccines. This has allowed people to resume some level of prepandemic activity.
Research revealed, among other things, that older age, male sex, obesity, and non-White race were more strongly associated with death from COVID-19 than from other causes and that smoking and a history of cancer or chronic liver disease were more strongly associated with death from causes other than COVID-19.1 Human diversity is what makes it impossible for one strategy—whether for addressing COVID-19 or cataract surgery and IOL selection—to be the right fit for everyone.
OCULAR DIFFERENCES
Biologic systems reflect complex genetic diversity that is expressed variably in the phenotype. Variation in ocular dimensions has been clearly demonstrated across geographic areas.2 Both interocular symmetry and statistically significant differences in ocular biometry are evident (Figure 1).3
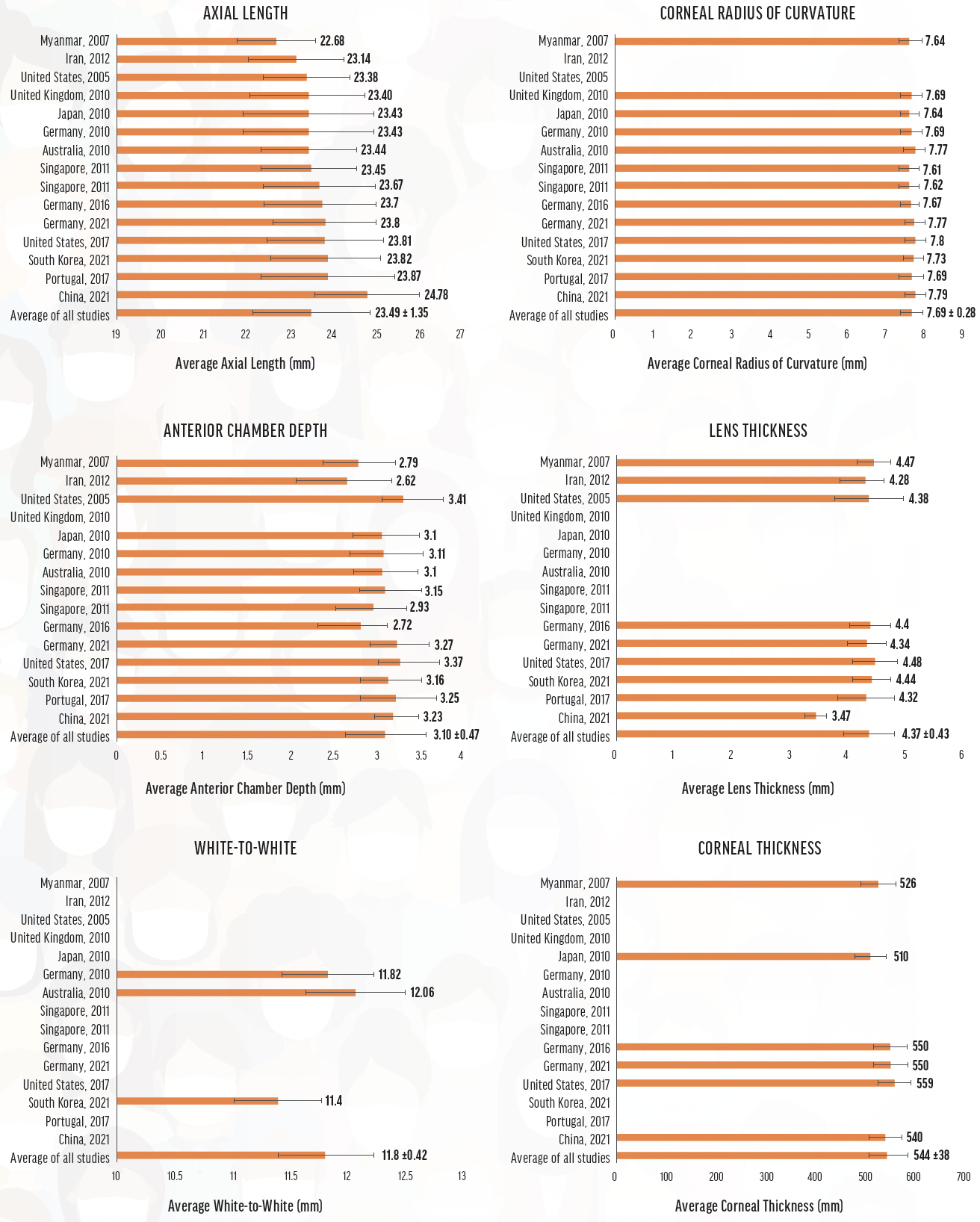
Figure 1. Global biometry averages and standard deviations derived from the studies used to compute global ocular metrics. Source: Ganesh D, Lin SR. Global metrics on ocular biometry: representative averages and standard deviations across ten countries from four continents. Eye (Lond). Published online February 21, 2022. doi:10.1038/s41433-022-01961-3
Patients with significant interocular differences are at increased risk of poor refractive outcomes after cataract surgery.4 Interocular differences contribute to variations in focal range, quality of vision, and the incidence and severity of dysphotopsias both among patients and between the eyes of the same patient. Surgeons cannot predict with certainty which patients and eyes will achieve a wider focal range, a lower level of dysphotopsias, or higher contrast sensitivity with the same IOL.
Figure 2 illustrates the variation in focal range achieved with two Johnson & Johnson Vision IOLs: the Tecnis Monofocal 1-Piece (model ZCB00) and the Tecnis Symfony extended depth of focus (EDOF) IOL. Of the 105 eyes in the monofocal IOL group, 45% achieved a distance-corrected intermediate visual acuity of 6/9 or better. Similarly, 64% of the 403 eyes that received the extended depth of focus IOL achieved 6/9 or better distance-corrected photopic near visual acuity (unpublished data).
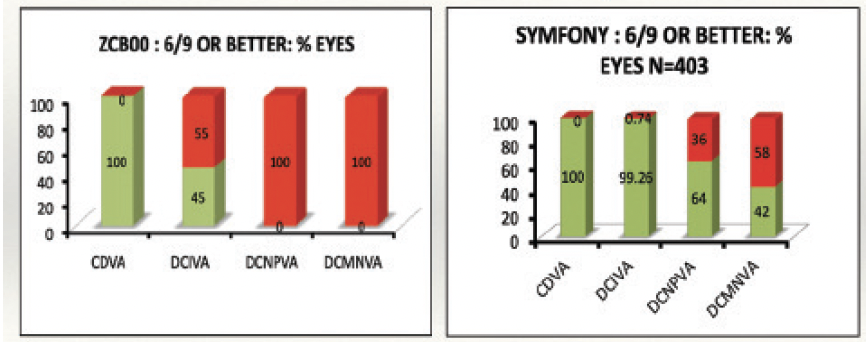
Figure 2. The median and range of distance-corrected monocular visual acuity with the Tecnis ZCB00 (N = 157) and Tecnis Symfony (N = 403) IOLs.
BINOCULAR CUSTOMIZATION
Most patients undergoing refractive cataract surgery desire good functional vision without spectacles. Meeting this expectation generally requires achieving a visual acuity of 6/9 or better, a seamless and continuous range of vision from infinity to 40 cm, minimal to no dysphotopsia, and maximal contrast sensitivity and quality of vision. Our challenge is to generate a pair of retinal images in the patient’s eyes that, after cortical processing and neural adaptation, achieve these objectives.
Binocular customization entails choosing an IOL for each eye that produces the best binocular vision in terms of focal range, contrast sensitivity, and dysphotopsia. It creates a set of retinal images that allows the binocular contrast sensitivity to be higher than monocular contrast sensitivity due to binocular fusion. I wrote about the topic in detail in the February issue of CRST Europe . The aim is to modulate the retinal image in each eye to optimize binocular functional vision. Binocular IOL customization is not a rule-based, mix-and-match approach to IOL selection. Instead, the process uses the outcome of cataract surgery on the first eye to generate a surgical plan for the second eye based on one of two methods of customization.
No. 1. The second eye receives an IOL with an optical design that differs from the IOL implanted in the first eye.
No. 2. The second eye receives the same IOL but a different refractive target is used.
Both approaches can address gaps in focal range, quality of vision, and dysphotopsia after surgery on the first eye.
AN OBJECTIVE ASSESSMENT TOOL
My colleagues and I developed the Panfocal-VA to objectively assess patient needs in terms of their focal range, quality of vision, and dysphotopsias. The functional vision tool is used preoperatively to evaluate and educate patients on their needs and select an IOL for the first eye. The assessment is repeated 1 to 2 weeks after surgery on the first eye to assess the patient’s vision.
The IOL for the second eye is chosen based on the assessment and the patient’s subjective feedback. The goals are to maximize range of vision without substantially compromising quality of vision and to minimize dysphotopsias. More on the use of the Panfocal-VA may be found in my February 2022 article mentioned earlier.
CONCLUSION
Understanding, measuring, and customizing the refractive solution for each eye of every patient is the future of refractive cataract surgery.
1. Bhaskaran K, Bacon S, Evans SJ, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. 2021;6:100109.
2. Ganesh D, Lin SR. Global metrics on ocular biometry: representative averages and standard deviations across ten countries from four continents. Eye (Lond). Published online February 21, 2022. doi:10.1038/s41433-022-01961-3
3. Albarrán-Diego C, Poyales F, López-Artero E, Garzón N, García-Montero M. Interocular biometric parameters comparison measured with swept-source technology. Int Ophthalmol. 2022;42(1):239-251.
4. Wai YZ, Ng QX, Adnan TH, Chong YY, Mohamad AS, Goh PP. Interocular optical biometry differences as predictors of postoperative cataract surgery refractive outcomes: a retrospective cohort study. Med J Malaysia. 2021;76(6):884-892.




