CASE PRESENTATION
A 73-year-old woman presents with decreased visual acuity. The patient underwent uneventful bilateral cataract surgery 9 years ago.
On presentation, BCVA is 20/50 OD with a manifest refraction of +0.50 +0.75 x 160º and 20/30 OS with a manifest refraction of -1.75 +0.50 x 165º. A slit-lamp examination of each eye shows an open capsule and dense glistenings throughout a one-piece hydrophobic acrylic IOL that is well positioned in the capsular bag (Figure). The glistenings are denser in the IOL in the right eye. The remainder of the examination, including corneal tomography, a dilated funduscopic examination, and macular OCT, are normal.
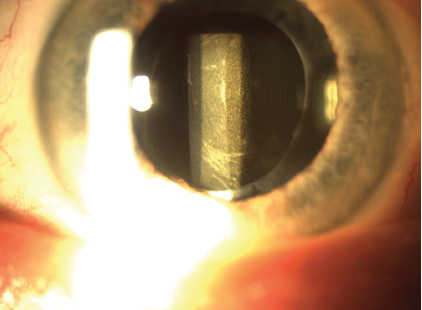
Figure. An IOL with dense glistenings is well positioned in the bag.
The patient states that there seems to be a film over her vision. She reports being told that she could undergo another Nd:YAG laser capsulotomy.
How would you counsel this patient? What surgical plan would you advise? If you would perform an IOL exchange, which IOL would you select, and what postoperative refraction would you target?
—Case prepared by Audrey R. Talley Rostov, MD

MICHAEL R. BANITT, MD, MHA
When approaching an unhappy patient, I try to determine the exact cause of the unhappiness and to make sure other causes have been addressed or ruled out. I would ask this patient if she is experiencing fluctuating vision throughout the day and blurred vision in the morning or evening. Is she using and wearing her current manifest refraction? To what extent is her visual status affecting her activities of daily living?
In the Figure, there appear to be concentric rings in the IOL as well as significant glistenings. If this is an extended depth of focus IOL, I would ask if she was happy with her vision at any point after surgery. I would also ask how she has used her eyes and glasses. For example, did she wear glasses 8 years ago? The history is important. Did she function well with the extended depth of focus IOL? With monovision? The answers to these questions would determine whether I offer an IOL exchange or recommend treating another issue that may allow her to be happy without an IOL exchange. I would likely direct her to buy a pair of glasses and use them before I offer surgery.
An IOL exchange is a last resort. Because this patient has already undergone an Nd:YAG laser capsulotomy, I would plan to implant a three-piece IOL. I prefer to have some stabilization of the IOL instead of simply placing it in the sulcus. Optic capture by the capsule would be attempted to stabilize the IOL, or the Yamane technique for scleral fixation would be employed. Suitable IOLs for optic capture include a SofPort (model LI61AO, Bausch + Lomb) and a Tecnis (model ZA9003, Johnson & Johnson Vision). I would have a CT Lucia 602 (Carl Zeiss Meditec) on hand in case scleral fixation becomes necessary.
The refractive target would likely be for distance, but a discussion with the patient about how she has used her eyes in the past and wants to use them in the future is required to make this determination. The conversation must take into account the 20/30 UCVA OS.

LISA K. FEULNER, MD, PHD
This situation is complicated but unfortunately not as uncommon as one might think. A number of variables must be considered before counseling this patient. Each lens appears to be a one-piece acrylic multifocal with notable glistenings. In each eye, the capsulorhexis is large, a round posterior capsulotomy is present, and there is obvious fibrosis of the anterior capsule. Additionally, both eyes have significant residual refractive errors with anisometropia. There is really no option other than an IOL exchange to address decreased BCVA caused by opacification of an IOL due to glistenings.
Before the IOL exchange, a lengthy discussion with the patient concerning her postoperative refractive expectations is required. Neither eye has good vision, but the glistenings and vision are worse in the right eye. Given the increased risk of performing an IOL exchange in the presence of an open capsule, I would encourage her to consider undergoing surgery on the right eye only in hopes of getting her to be visually functional with both eyes using glasses or contact lenses. I would therefore recommend an IOL exchange for a three-piece monofocal lens with a target of -0.50 D in the right eye. I would discourage the use of a multifocal IOL placed in the bag or a three-piece multifocal lens fixated in the sulcus because, technically, it is more difficult to achieve a favorable refractive outcome with either of these strategies. Moreover, an IOL exchange would be necessary in the second eye.
The open posterior capsule increases the risks of vitreous prolapse and extension of the posterior capsulotomy. In addition, after 9 years, there are likely significant fibrotic adhesions around the optic and haptics, which will make removal of the IOL more difficult and increase the chance of zonular destabilization. I would plan for an in-the-bag exchange but be prepared to place the lens in the sulcus or even the anterior chamber if necessary.
Intravenous mannitol would be administered 30 minutes before surgery to shrink vitreous volume and reduce posterior pressure. A new sideport incision and a temporal clear corneal incision would be made at the limbus with a 2.7-mm keratome. A dispersive OVD (Viscoat, Alcon) would be instilled to protect the cornea and maintain the chamber. Using a bent 25-gauge needle, the capsule would be lifted mechanically from the optic as close to the optic-haptic junction as possible, and an OVD would be injected gently to create space for a 27-gauge cannula and to try to separate the optic from the remaining posterior capsular bag. A dispersive OVD would be injected behind the optic to tamponade the vitreous and prevent rupture of the hyaloid membrane.
After the optic is free, the haptics would be dissected from the capsule. In this case, the ends of the haptics are likely to be encapsulated and tightly fibrosed to the capsule, so it may not be possible to free them. If necessary, the optic would be severed from the haptics at their junction with microincision forceps, the optic or the entire lens would be floated into the anterior chamber, and a three-piece IOL would be inserted into the bag or sulcus. Once the new lens is in place, the internal wound would be enlarged to 2.8 to 3.0 mm. Then, the original IOL would be grasped at the haptic-optic junction and removed through the wound using a hand-over-hand technique.
Sometimes it is necessary to bisect the IOL optic partially to facilitate its removal. If vitreous prolapses at any point, an anterior vitrectomy would be performed. Once the new lens is securely in place, the anterior chamber is clear of vitreous, and the OVD has been removed, a miotic agent would be injected, and the wound would be secured with hydration and a suture.
The patient would receive acetazolamide (Diamox, Duramed Pharmaceuticals) 500-mg sequels in recovery, before bedtime, and on the morning after surgery.

LAWRENCE WOODARD, MD
Performing an IOL exchange after an Nd:YAG laser capsulotomy requires careful planning and a detailed discussion and informed consent with the patient. Silicone IOLs have been shown to have a lower incidence of glistenings than acrylic IOLs.1 I would explain to this patient that exchanging the original IOL for a silicone SofPort IOL will likely improve her quality of vision. I would emphasize, however, that this procedure is not without risk because of the open posterior capsule. Another Nd:YAG laser capsulotomy will not improve her symptoms.
A Sinskey hook would be used to dissect the anterior capsule from the anterior optic surface. If this maneuver is unsuccessful, a 27-gauge needle oriented bevel down on the OVD syringe can be used to separate the capsule from the optic, while OVD is gently expressed to initiate viscodissection. A 30-gauge cannula would then be used to viscodissect the area around both haptics and to expand the capsular bag. A Sinskey hook can be useful for gentle attempts at freeing haptics from the capsular bag. Microforceps and microscissors (both from MicroSurgical Technology) would then be used to cut the optic in half and remove the pieces. If the haptics are not easily freed from the bag, they would be amputated to free the optic without placing further stress on the bag.
The haptics of the new IOL would be placed in the ciliary sulcus, and the optic would be captured with the anterior capsule. The power of the IOL would be decreased by 0.50 D from the in-the-bag power calculation.

WHAT I DID: AUDREY R. TALLEY ROSTOV, MD
After a lengthy discussion with the patient, the one-piece hydrophobic acrylic IOL was exchanged for a three-piece SofPort lens (model LI61AO). A sideport incision was created with a diamond blade, and a 3-mm temporal incision was made with a diamond keratome. A dispersive OVD (Viscoat) was injected into the anterior chamber and posterior to the IOL. The IOL was carefully elevated out of the bag and into the anterior chamber with Kuglen and Sinskey hooks, and an additional amount of OVD was instilled as necessary. Patience and care were required to remove the IOL from the bag. Microscissors and microforceps (both from MicroSurgical Technology) were used to cut the IOL in several places and then twist and turn it out of the 3-mm temporal incision.
A limited bimanual vitrectomy was performed to prevent vitreous prolapse into the anterior chamber. A cohesive OVD (ProVisc, Alcon) was injected, and the SofPort IOL was placed in the sulcus, its optic captured through the anterior capsulorhexis. I was prepared to perform scleral fixation of the lens using the Yamane technique if capsular support had been inadequate.
Postoperatively, the patient did well. Uncorrected distance visual acuity was 20/25 with a minimal refraction of -0.50 +0.50 x 170º. She reported a big improvement in her vision and has deferred undergoing an IOL exchange in the left eye.
1. Oshika T, Ando H, Inoue Y, et al. Influence of surface light scattering and glistenings of intraocular lenses on visual function 15 to 20 years after surgery. J Cataract Refract Surg. 2018;44(2):219-225.




