Robotic systems have been widely incorporated into many surgical applications because of their higher precision, greater maneuverability, and potential for improved sensing capabilities compared with manually performed surgical procedures. The pace at which robotic systems have been integrated into ophthalmic surgery, however, has lagged behind that of other surgical fields. This difference can be attributed to the unique advantages of manual ophthalmic surgery, including direct visualization of the surgical workspace and unhindered maneuverability of intraocular instrumentation. These advantages have eclipsed the apparent benefits of incorporating robotics into the ophthalmic surgical theater. Further, significant progress has already been made toward improving surgical outcomes through the incorporation of state-of-the-art digital microscopes, advanced fluidics control during phacoemulsification, and laser cataract surgery.
Nevertheless, manual surgical procedures are constrained by the physiologic limitations of the human surgeon. Precise physical manipulation of intraocular tissue is hindered by inherent hand tremor, an inability to sense forces below those of human tactile perception, and a lack of sufficient depth perception to resolve microstructures or identify tissue planes. These limitations do not hinder a robotic system to the same degree. This has inspired extensive research and development during the past 2 decades.
Here we highlight the beginnings of robotic surgery in ophthalmology, describe how robotic surgery is being used today, and discuss its future potential for cataract surgery.
BEGINNINGS
Since the early 1990s, dozens of robotic systems intended for intraocular surgical use, ranging from handheld to articulated robotic systems, have been prototyped and evaluated in research laboratories across the world. The Micron, a fully actuated handheld robotic instrument developed by Carnegie Mellon and Johns Hopkins universities, has been shown to reduce hand tremor to provide a smooth, scaled motion during surgical procedures.1 A demonstrated application includes retinal vein cannulation on artificial vein models.
A research group at Vanderbilt University incorporated a B-mode OCT probe into a pair of microforceps to form a handheld surgical device that allows visualization of the membrane surface during epiretinal membrane–peeling operations.2 The researchers showed that the tool-to-membrane distance could be known in real time from the OCT visualization and that this knowledge improved a surgeon’s performance during membrane peeling in ex vivo goat eyes.
In terms of articulated robotic systems, a research group at the Technical University of Munich demonstrated a mechanism small enough to be mounted to a patient’s head.3 This device was used to perform a range of intraocular procedures, including subretinal injection and deep anterior lamellar keratoplasty. Recent work incorporates OCT-based needle segmentation techniques to expand the boundaries of autonomous surgical procedures.
To our knowledge, the only robotic system to be developed specifically for cataract surgery is the Intraocular Robotic Interventional and Surgical System (IRISS) from the University of California, Los Angeles (Figure 1).4 Presented in 2013, the IRISS was the first robotic system to demonstrate simultaneous use of two surgical instruments, teleoperated capsulorhexis, and an entire cataract surgery on ex vivo pig eyes.
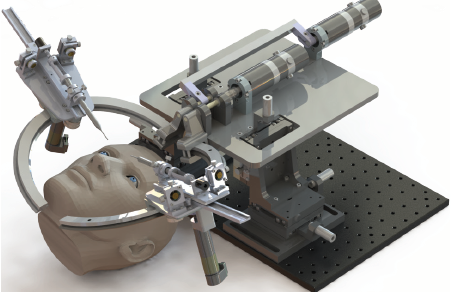
Figure 1. The IRISS from UCLA.
Courtesy of UCLA
More recently, the IRISS was integrated with an OCT system to perform a range of autonomous surgical procedures, including partially automated lens extraction.5 In that work, preoperative OCT scans were used to generate a lens-extraction trajectory, and intraoperative OCT scans localized around the tip of the I/A handpiece were displayed to the surgeon to enable real-time anatomical evaluation. On an ex vivo pig eye model, posterior capsular rupture (PCR) was avoided in all trials, and nearly complete lens extraction was achieved with the IRISS.
CURRENT STATUS
To date, the Preceyes Surgical System (Preceyes) is the only robotic surgery system dedicated to ophthalmology to become commercially available. The system consists of a joystick-like motion controller used as an input by the surgeon and an instrument manipulator that mounts to the surgical instrument (Figure 2). In 2018, the Preceyes was used to perform retinal membrane peeling and subretinal injection in humans.6 With this system, a high degree of tool-tip positional precision can be obtained, and virtual boundaries can be imposed to restrict unwanted movement and prevent iatrogenic retinal trauma. The Preceyes group continues to publish research results with their system, and at least three devices are currently being used in hospitals across Europe (for more information on Preceyes, see Making Eye Surgery Smarter and Better).
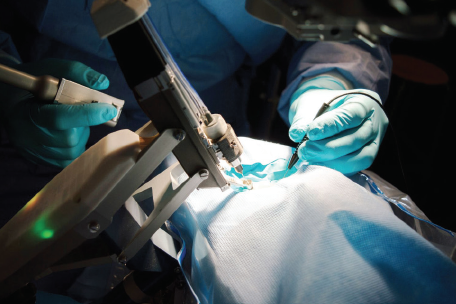
Figure 2. The Preceyes Surgical System.
Courtesy of Preceyes
Making Eye Surgery Smarter and Better
By Sean Ianchulev, MD, MPH

Interventional precision is the goal of any surgery but particularly of ophthalmic microsurgery. Innovation and improvement in surgical precision, however, have been slow in the past 5 decades. Manual surgical instruments have remained the same for the most part. Breaking new ground in surgical treatment requires an extension of the manual skill set to achieve higher precision and stability so that microlevel structures become accessible, new procedures can be developed, or the outcomes of existing procedures can be improved.
Even the best surgeons are prone to tremor and limits of manual dexterity. Manual surgical precision has a root mean square amplitude of tremor of 180 µm and a peak-to-peak vector magnitude of 100 µm.1 A 100-µm–level threshold is inadequate when dealing with anatomic structures that are within the range of this variability. For the anatomy of the subretinal space, the retinal vasculature, or Schlemm canal, micron-level precision is required.2
The Preceyes Surgical System (Preceyes) is the first robotic assistant that can improve the precision threshold of manual surgical dexterity 20-fold.3
THE SYSTEM
Preceyes offers 5-µm–level precision, tremor control, stabilization, and semiautomation. The system has the CE Mark and is used clinically for vitreoretinal surgery. It can be used to increase the precision of essential interventions such as internal limiting membrane peeling and subretinal injection.
The technology was developed as an extension of manual instruments; it is fully integrated into the standard existing surgical setting. Preceyes consists of a manipulator to hold a surgical instrument and a joystick that the surgeon uses to control the movements of the instrument. In practice, the manipulator copies the movements of the surgeon, leaving the surgeon in full control of the instrument and the surgery. The device scales movements and filters tremors of the surgeon, thereby increasing precision and stability.
Preceyes is a platform technology. It can therefore be applied across a spectrum of ophthalmic procedures. This year, the company partnered with a surgical innovation group at New York Eye and Ear Infirmary of Mount Sinai to develop and demonstrate the platform application for the anterior segment with the first high-precision robot-assisted gonioscopy intervention module. The system has been deployed at New York Eye and Ear (Figure) in preparation for FDA trials to bring the technology to the US market.

Figure. Dr. Ianchulev (center in the first image) and colleagues at New York Eye and Ear experiment with the Preceyes system.
A LOOK AHEAD
The implementation of robotic assistance requires a distinct shift in surgical training and skill set. Robotics and smart instrumentation can make super surgeons, but ophthalmologists must learn to embrace a new surgical interface that will come between them and the patient. Like a gaming console or a cockpit, the surgical tool set will become more sophisticated.
The timing of this evolution could not be better. If advances in surgical tools are combined with digital imaging, virtual reality, smart sensing, automation, and AI, Preceyes may be just a precursor of a completely different future—the surgical metaverse!
1. Singhy SPN, Riviere CN. Physiological tremor amplitude during retinal microsurgery. Paper presented at: Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference (IEEE Cat. No.02CH37342). April 21, 2002; Philadelphia.
2. Riviere CN, Jensen PS. A study of instrument motion in retinal microsurgery. Paper presented at: Engineering in Medicine and Biology Society, Proceedings of the 22nd Annual International Conference of the IEEE. July 23-28, 2000; Chicago.
3. Roizenblatt M, Edwards TL, Gehlbach PL. Robot-assisted vitreoretinal surgery: current perspectives. Robotic Surgery: Research and Reviews. 2018;5:1-11.
The first-in-human experience with the robotic laser FemtoMatrix (Keranova) was first presented in 2019.7 The system can be used to photoemulsify the cataractous lens. The company reports that the FemtoMatrix may be used to perform about 80% of the surgical procedure without human intervention.8 (For more information on Keranova, see Workflow Integration With the Keranova Workstation.)
Workflow Integration With the Keranova Workstation
By Michael Brownell

Significant challenges in workflow result from current laser cataract systems because the femtosecond lasers cannot be efficiently integrated into the OR. They are large and immobile, requiring additional staff and space in most clinics to facilitate workflow. Patients and staff must move to the laser instead of the technology’s coming to them, which would decrease the laser cataract procedure time and the number of steps required.
Further, phacoemulsification is still required for virtually every eye that undergoes laser cataract surgery. This further complicates workflow and puts the femtosecond laser in the role of an auxiliary rather than an integrated technology.
NEW SOLUTIONS
These issues have limited the adoption of laser cataract surgery, reduced penetration of femtosecond lasers into the market, and frustrated surgeons. In response, Keranova has built new solutions into its system.
Robotic docking arm. A docking arm designed to automatically move in and out of the surgical space may be a game-changer for OR integration. The laser delivery head at the end of the robotic arm shares space with the surgical microscope, so all steps of the cataract procedure can be executed without requiring the patient and surgeon to move (Figure 1). Moreover, the mobile chassis can facilitate various layouts and workflows in the OR.
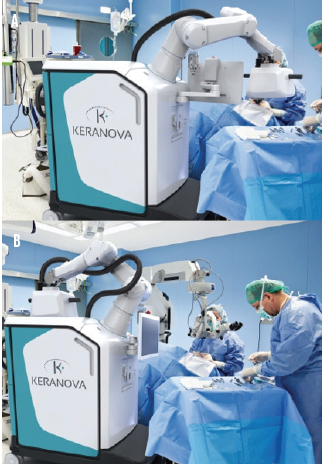
Figure 1. The robotic arm docked on a patient for a laser procedure in the OR (A) and back in the home position (B), which creates space for the surgical microscope for aspiration and lens implantation.
The robotic arm automatically moves into position over the patient for docking when the surgeon is ready and returns to the home position over the system’s chassis when not in use. A robotic-assisted light-touch movement by the surgeon docks the system onto the patient’s eye. Then the entire laser procedure for the lens, capsule, and cornea runs automatically according to the preoperative treatment plan and any real-time adjustments made by the surgeon. Future versions of this technology could include fully automated robotic docking and undocking and 3D visualization systems for aspiration and IOL implantation.
Photoemulsification. This innovation enables the laser to do complete lens emulsification with all densities of cataracts for direct aspiration. The Keranova system uses laser wavefront modification to create scanning beams with multiple focused spots and beam shapes. The system also maps the laser energy threshold throughout the inhomogeneous cataract lens at the beginning of the procedure. This is key to ensuring complete incisions in all scanning planes within the lens volume while minimizing the amount of gas created (Figure 2).
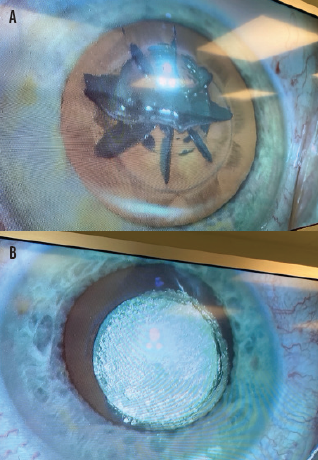
Figure 2. Large uncut zones and gas volume after lens fragmentation with a currently available femtosecond laser (A) and the lens of the contralateral eye after photoemulsification with the Keranova workstation (B).
In clinical studies, the Keranova system emulsified even very dense (≥ grade 5) cataract lenses into a homogeneous volume of 200-µm cubes that can be aspirated without phaco energy (Figure 3). Photoemulsification has resulted in laser energy and beam pattern optimization for each lens for fast, efficient removal.1
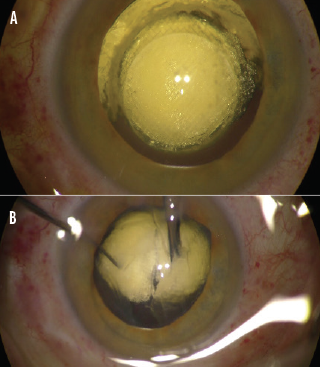
Figure 3. A grade 5 cataract is photoemulsified (A), and aspiration begins (B).
System integration. Aspiration fluidics and phaco assistance are integrated into the system to increase workflow efficiency. The novel scanning optical system is designed to minimize the focused spot size throughout the scan volume from the anterior cornea to the posterior lens. Furthermore, quality LASIK flaps have been created in the lab in a few seconds, showing it to be a true cataract and refractive workstation.
CONCLUSION
With the Keranova workstation, procedural automation and robotics are bringing greater efficiency to cataract surgical workflow. Clinical studies for the CE Mark are expected to be completed this year.
1. Brownell M. Keranova. Paper presented at: OIS European Innovation Showcase; September 23, 2020; virtual event.
The first group to demonstrate in-human, robot-assisted retinal vein cannulation was Mynutia, a spinoff of the Catholic University of Leuven, Belgium. The surgeon and the comanipulated robotic system simultaneously hold the same surgical instrument (Figure 3). In the 2018 phase 1 clinical trial, an anticoagulant was injected into the retinal veins of four patients with retinal vein occlusion for up to 10 minutes.9 Development of the system continues, and the company is attempting to commercialize the technology.
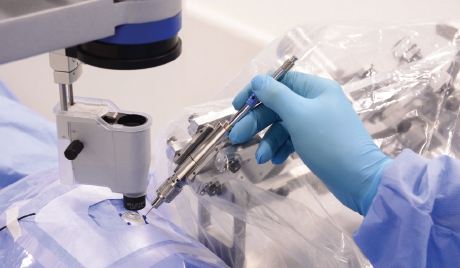
Figure 3. The Mynutia Surgical System.
Courtesy of Mynutia
Research on the IRISS continues at the University of California, Los Angeles. The goal is to develop a system capable of performing fully automated cataract surgery. The most recent work demonstrated partially automated polishing of the posterior capsule in ex vivo pig eyes using transpupillary OCT scans of the anterior segment.10 In related research, a deep learning–based method was used to guide an I/A handpiece to autonomously extract lens fragments from ex vivo pig eyes.11 This work provides a foundation for autonomous cataract surgery guided by AI.
A few startup companies—AcuSurgical, Cambridge Consultants, Forsight Robotics, and Ophthorobotics—have received seed funding for their unique visions of intraocular robotic surgical systems. These companies are reported to be working toward clinical trials and the CE Mark. No evaluations of these systems, however, are known to exist. (For more information on AcuSurgical, see The AcuSurgical Cockpit.)
The AcuSurgical Cockpit
By Michael Brownell

During ophthalmic surgery, the surgeon’s hands make small and critical instrument gestures while the surgeon’s body remains in specific positions for long periods and the surgeon’s eyes look through a microscope. An innovative approach is being developed to increase the precision, stability, and repeatability of procedures with user-friendly robotics that function as an extension of the surgeon’s skill. This type of solution may give rise to new procedures and streamline existing ones—all with the surgeon in comfortable ergonomic positions and flexible locations. Robotics can also facilitate advanced visualization technologies, improve workflow, and optimize space in the OR.
THE SETUP
AcuSurgical recently raised €5.75 million in series A financing to advance its robotic ocular microsurgery platform that surgeons will pilot through procedures by leveraging the classic skills of their profession and common instruments. The cockpit for this surgical pilot is a console with physical styluses that have haptic feedback so that surgeons can feel the procedure and control the motion directly in real time while receiving image guidance using 3D visualization and OCT sensing.
The one or two surgical arms of the AcuSurgical cockpit adjacent to the patient have tool adapters for the various familiar surgical instruments. This setup provides surgeons with instinctive instrument tip movement and control in three dimensions inside the eye. The system therefore adds effective and practical robotic technology to surgeons’ existing techniques and knowledge. Over time, they can learn together how to optimize and innovate procedures.
A NEW FRONTIER
Ramin Tadayoni, MD, PhD, is a professor of ophthalmology and the head of the department of ophthalmology at Université de Paris. After a recent preclinical retinal session in AcuSurgical labs, Dr. Tadayoni remarked, “Retinal imaging today has reached micron resolution. If surgery comes to this resolution, a new frontier will open.” Procedures such as gene and cell therapy could be made practical through this type of robotic surgery.
Robotics is also well known for making consistently performed processes more efficient through movement algorithms, motion scaling, and data processing. As a result, steps can be streamlined, human fatigue and error reduced, the user experience improved, and outcomes made more consistent across different surgeons and patients. High-volume ophthalmology practices and areas with high patient-to-surgeon ratios could also take advantage of the efficiencies of a robotic approach. Multispecialty practices could benefit from the flexibility and adaptability of high-quality robotic surgeries for a variety of indications, including cataract removal and IOL implantation. Robotics could effectively complement the procedural automation enabled by femtosecond lasers.
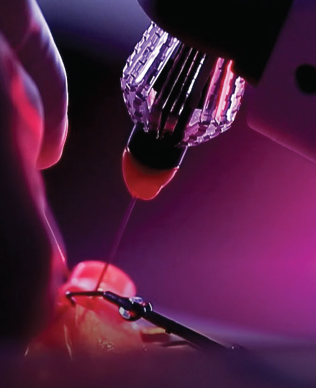
Figure. The AcuSurgical robot is used to perform a procedure at a recent wet lab.
CONCLUSION
Ophthalmologists tend to enjoy technology, and the field of robotics is just starting to put surgeons into the pilot’s seat.
POTENTIAL FOR CATARACT SURGERY
The integration of robotic systems could improve cataract surgery in several ways.
Access. The number of trained cataract surgeons is inadequate to meet current demand, and this shortage is expected to worsen as the population ages and the prevalence of cataracts increases. A robotic surgeon could help increase health care access by performing routine surgery on patients with cataracts that are not complex.
Efficiency. The seamless integration of all surgical steps into a single system could improve surgical flow and decrease operation time compared to manual surgery. Further, it would not require relocation of the patient, as is currently necessary for laser cataract surgery.
Safety. If we add up all the peri- and postoperative complications, including refractive errors, an estimated 50% of patients will experience suboptimal outcomes: Posterior capsular opacification rates are around 30% at 5 years,12 PCR rates are around 5%,13 and suboptimal refractive outcome rates are around 30%.14 By standardizing treatment, a robotic system could enable every surgeon to improve outcomes.
The greatest surgical risk originates with cataract removal. For example, PCR remains a common complication largely because of the difficulty of sensing the location of the posterior capsule during phacoemulsification, irrigation, and aspiration. Novel technologies capable of sensing the posterior capsule (eg, intraoperative OCT) exist. Their data, however, are not well integrated and are difficult for a human surgeon to interpret. Recent advances in computer vision techniques allow a robotic system to be trained to make sense of these data and safely guide the tool. It could do this with greater resolution and a faster response time than a human surgeon is capable of, thereby reducing the occurrence of surgical complications that arise from improper tool positioning, excessive aspiration forces, and ultrasound power.
Another potential future benefit is reducing posterior capsular opacification through complete polishing of the entire capsular bag. Currently, human surgeons are limited by an inability to visualize the lens equator, the difficulty of sensing the position of the posterior capsule relative to the tool, and inadequate control of aspiration forces and response times. Robotic solutions include the incorporation of novel visualization technologies capable of imaging the capsule equator, robotically guiding tool positioning, and high-resolution control of aspiration forces with fast response times. If well integrated, these capabilities could allow safe and complete polishing of the entire capsular bag without increasing the risk of PCR.
Automation. Augmentation of surgical decisions through the incorporation of AI is a major anticipated development. Robotic cataract surgery is especially well suited to automation because the surgical protocol is remarkably consistent and comprises the same specific, routine tasks. An existing example is the use of a femtosecond laser to create the corneal incision(s), perform the capsulotomy, and fragment the lens before manual extraction.
A more ambitious but not infeasible application is to automate the entire surgery, effectively eliminating the requirement for a surgeon to hold the surgical instruments. Such a goal can only be achieved with improved feedback from OCT or another imaging modality closely integrated with a robotic system, and AI guidance would be required to make surgical decisions with limited or no input from a human surgeon.
CHALLENGES
Despite increased accuracy and precision, tremor filtering, improved visualization, and other potential benefits of robotic cataract surgery, significant obstacles remain to the technology’s incorporation into routine practice.
The advent of laser cataract surgery in 2010 is a case in point. Even a decade after its inception, this technology is not yet established as a standard technique because of added cost, questionable added value, and interruption of the surgical flow. These issues and others must be addressed if intraocular robotic surgical systems are to become commonplace in ORs.
CONCLUSION
The full potential of surgical robotics in cataract surgery lies in developing an integrated robotic system that adds significant value over current manual surgical techniques. Improved knowledge of surgical instrument positioning through the use of OCT and high-precision robotic systems has the potential to reduce or eliminate anatomical damage such as PCR. Nevertheless, improvements to current robotic systems and their acceptance in general practice are required before cataract surgery robotic systems can be widely deployed.
1. Yang S, MacLachlan RA, Riviere CN. Manipulator design and operation for a six-degree-of-freedom handheld tremor-canceling microsurgical instrument. IEEE ASME Trans Mechatron. 2015;20(2):761-772.
2. Yu H, Shen JH, Shah RJ, Simaan N, Joos KM. Evaluation of microsurgical tasks with OCT-guided and/or robot-assisted ophthalmic forceps. Biomed Opt Express. 2015;6(2):457-472.
3. Nasseri MA, Eder M, Nair S, et al. The introduction of a new robot for assistance in ophthalmic surgery. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:5682-5685.
4. Rahimy E, Wilson J, Tsao TC, Schwartz S, Hubschman JP. Robot-assisted intraocular surgery: development of the IRISS and feasibility studies in an animal model. Eye (Lond). 2013;27(8):972-978.
5. Chen CW, Lee YH, Gerber MJ, et al. Intraocular robotic interventional surgical system (IRISS): semi-automated OCT-guided cataract removal. Int J Med Robot. 2018;14(6):e1949.
6. Edwards TL, Xue K, Meenink HCM, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018;2:649-656.
7. Stodulka P. Keranova robotic arm femtomatrix superfast cataract surgery. Paper presented at Ophthalmology Innovation Summit 2019; May 2, 2019; San Diego.
8. Technology. Keranova. Accessed June 9, 2021. https://www.keranova.fr/en/technology/
9. Gijbels A, Smits J, Schoevaerdts L, et al. In-human robot-assisted retinal vein cannulation, a world first. Ann Biomed Eng. 2018;46(10):1676-1685.
10. Gerber MJ, Hubschman JP, Tsao TC. Robotic posterior capsule polishing by optical coherence tomography image guidance. Int J Med Robot. Published online February 27, 2021. doi:10.1002/rcs.2248
11. Shin C, Gerber MJ, Lee YH, et al. Semi-automated extraction of lens fragments via a surgical robot using semantic segmentation of OCT images with deep learning – experimental results in ex vivo animal model. IEEE Robot Autom Lett. 2021;6(3):5261-5268.
12. Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998;105(7):1213-1221.
13. Bhagat N, Nissirios N, Potdevin L, et al. Complications in resident-performed phacoemulsification cataract surgery at New Jersey Medical School. Br J Ophthalmology. 2007; 91(10):1315-1317.
14. Durr GM, Ahmed IIK. Intraocular lens complications: decentration, uveitis–glaucoma–hyphema syndrome, opacification, and refractive surprises. Ophthalmology. 2020;S0161-6420(20)30637-0.