All foldable acrylic IOLs share one highly valued characteristic: They allow surgeons to use small incisions to implant them, leading to rapid healing times. Acrylic IOL materials can be divided into two major classes. The first, hydrophilic, includes materials that are attracted to water, and the second, hydrophobic, materials that repel water. Finer distinctions among hydrophobic and hydrophilic acrylic IOLs have been researched, reported, debated, and debunked for decades, but both types of material are still widely used.
These broad classifications—hydrophobic and hydrophilic—may soon be changing as new materials and surface treatments for IOLs are introduced. Some of these advanced materials are already on the market, while others are in investigative stages. This article outlines the distinctions between hydrophobic and hydrophilic materials that lead some surgeons to prefer one type over the other and looks ahead to the advances in IOL materials that may soon lead to changes in the parameters of these two broad terms.
SURGEON PREFERENCES
Hydrophobic acrylic IOLs are the market leaders among US surgeons, but hydrophilic acrylic IOLs continue to enjoy a healthy following outside the United States, as well as use by some US surgeons. The latest market data indicate that foldable hydrophobic acrylic IOLs represent 56% of the IOL market globally and hydrophilic acrylic IOLs represent 29% (Figure).1
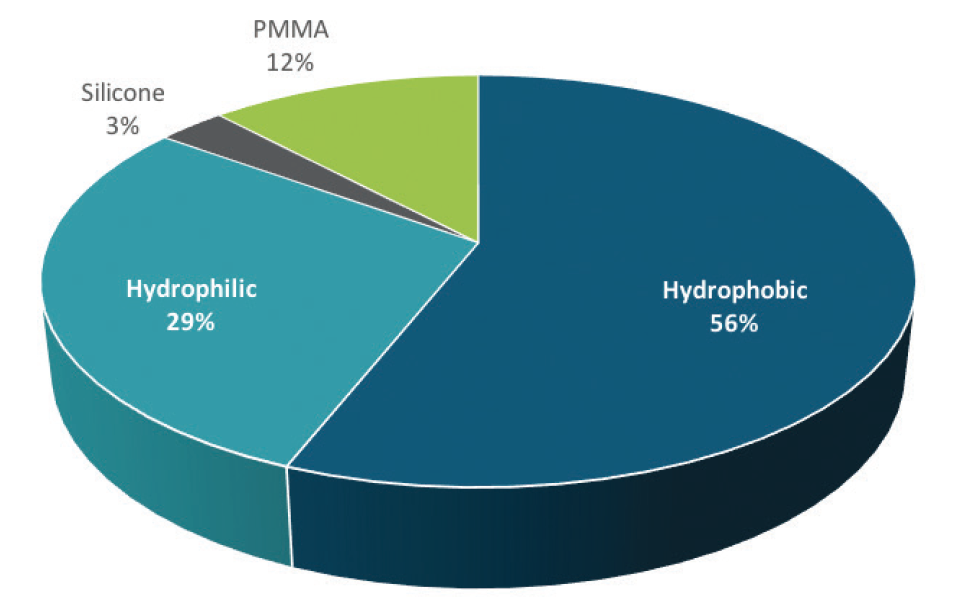
Figure. 2017 global IOL market share by optic material.
(Source and image courtesy of Market Scope)
“IOL material preferences continue to move away from PMMA and silicone and toward hydrophobic and hydrophilic” acrylic materials, William Freeman, author of Market Scope’s 2018 IOL Report: A Global Market Analysis for 2017 to 2023,1 told CRST.
“Hydrophilic acrylic IOLs are not very popular in the United States, but they are quite popular in other markets, such as in Europe,” said Liliana Werner, MD, PhD, a Professor of Ophthalmology and Visual Sciences and the Codirector of the Intermountain Ocular Research Center at the John A. Moran Eye Center of the University of Utah in Salt Lake City.
Randall J. Olson, MD, a Professor and the CEO and Chair of Ophthalmology and Visual Sciences also at the Moran Eye Center, added that hydrophilic acrylic materials are easier to work with and to sterilize than hydrophobic materials. He said the popularity of hydrophilic IOLs in Europe “relates to the ease of approval in the European Union, which results in many smaller companies having products in niche markets.”
According to Florian T.A. Kretz, MD, preferences for hydrophobic or hydrophilic acrylic IOLs vary across Europe. “It is not only in the United States where hydrophobic material is more popular than hydrophilic. In Europe, the preference varies by country and even state. In Germany, for instance, hydrophobic material has proven its stability over decades—even the possibility of glistenings has roughly been extinct over the past couple of years.” Dr. Kretz is in private practice in Germany and a member of the Department of Ophthalmology at the University of Heidelberg.
MATERIAL DIFFERENCES
Some general distinctions can be made between hydrophobic and hydrophilic acrylic IOL materials. The main difference is the water content of the materials. Nonpolar molecules that repel water molecules are said to be hydrophobic; molecules that form ionic or hydrogen bonds with water molecules are said to be hydrophilic. Hydrophilic IOL materials, also known as hydrogels, have water content ranging from 18% to as high as 38%.2 Historically, hydrophobic IOL materials have had low water content, generally less than 1%.2 However, these boundaries are changing with the advent of some recently developed IOL materials that may have advantages over more traditional hydrophobic materials.
“In recent years, hydrophobic acrylic materials with water content higher than the typical less than 0.5% have been developed,” Dr. Werner said. “These materials were found to be glistening-free in vitro and in vivo.”3
IOLs that incorporate higher water-content hydrophobic materials include the »enVista MX60 lens (Bausch + Lomb), with water content of approximately 4%,4 and the »FineVision HP (PhysIOL), with water content of approximately 4.9%.5 In 2017, outside the United States, Alcon launched the Clareon IOL, made of a hydrophobic acrylic material with 1.5% water content.6
Going in the opposite direction, hydrophilic materials with higher water content are being introduced, Dr. Kretz said. “If you take a close look into the latest hydrophobic materials, the water content is rising because it makes the implants more flexible, especially for sub–2-mm delivery,” he said.
The boundaries between hydrophilic and hydrophobic materials are narrowing, Dr. Kretz said, and soon “it might be necessary to reevaluate material definitions.”
PCO PERSPECTIVES
Hydrophilic IOLs historically have been associated with higher rates of posterior capsular opacification (PCO) than hydrophobic IOLs.
Studies have suggested that one explanation for the difference between PCO rates in hydrophobic and hydrophilic material IOLs may involve the ability of hydrophobic materials to adhere to collagen membranes, resulting in tight apposition of the lens to the posterior capsule. One 2005 study found greater PCO development at 1 year in hydrophilic than hydrophobic one-piece IOLs, but the investigators were not able to rule out the effect of IOL design in this study.7
A 2017 meta-analysis comparing PCO prevalence in hydrophobic and hydrophilic IOLs found that hydrophobic IOLs had an overall lower rate of PCO and Nd:YAG laser capsulotomy, although this difference was not associated with superior visual acuity8; an earlier meta-analysis on the same topic reached similar conclusions.9
A large retrospective study in the United Kingdom, based on electronic health records data for more than 50,000 eyes, found lower rates of PCO incidence and Nd:YAG laser posterior capsulotomy with hydrophobic AcrySof (Alcon) IOLs compared with non-Alcon hydrophobic and hydrophilic IOLs.10
Dr. Werner noted that differences in PCO rates “may not be only a material issue, but mostly a design issue.” The introduction of posterior square edge optic designs in both hydrophobic and hydrophilic IOLs has decreased the incidence of PCO with both types of materials. Studies show, however, that all square edges are not the same, and hydrophilic acrylic IOLs as a group have edges that are less square than those of hydrophobic acrylics.11,12
CALCIFICATION AND GLISTENINGS
PCO can be resolved in short order with a trip to the Nd:YAG laser. However, longer-term issues that cannot be so easily resolved have been reported with both hydrophobic and hydrophilic IOL materials. Calcification is a problem seen in some hydrophilic IOLs, and glistenings in hydrophobic IOLs.
Calcification remains an issue despite advances in materials and design of hydrophilic IOLs, Dr. Werner said.13 “There has been a significant number of explantations of hydrophilic acrylic lenses in Europe in recent years, for example IOLs manufactured by a company in Germany,” she said.14 “While calcification may not occur in significant numbers with some other hydrophilic acrylic lenses, we have observed that calcification may occur with any type of hydrophilic acrylic lens after procedures using repeated intracameral injections of gas or air, such as some types of endothelial keratoplasty. We have also observed that this may occur after any secondary surgical procedure, such as vitrectomy or glaucoma surgery.”15,16
Because hydrophilic materials have a higher water content than hydrophobic materials, there is a greater chance for other molecules to enter the material using water as a carrier, according to Dr. Kretz. This may be what leads to calcification. The more important question, he said, is to determine why this happens.
On the other hand, he continued, hydrophobic IOLs have had issues with glistenings, which are microvacuoles or fluid inclusions in the polymeric material. “Hydrophobic material can have problems and cause optical disturbances due to glistenings. Other opacifications in hydrophobic material seem to be reversible,” he said.17
Some studies have reported that glistenings have no impact on visual acuity.18,19 Dr. Werner noted that the effect of glistenings on postoperative visual function remains controversial, but resultant IOL explantation has rarely been reported.3
Dr. Kretz said that any surgery that can dry out a hydrophilic IOL material can potentially be a cause of calcification. He recommended performing Descemet membrane endothelial keratoplasty with an add-on IOL, which can later be removed, in front of the primary implant to protect it.
“From my point of view, the water content in the material carries the molecules causing calcification,” he said. “If the surface of the IOL and the material dry out—during vitrectomy, anterior segment procedures under air, or anterior segment fillings with high-surface-tension viscoelastic—the typical fluid-exchange mechanism in the material does not work anymore, so concentration rises and causes opacifications. But not every patient needs a secondary surgery, and surgeries can be adapted.”
A research group at Kingston University in the United Kingdom suggested one such adaptation. They investigated the links between Descemet-stripping endothelial keratoplasty (DSEK) and IOL calcification and concluded that a simple adjustment to the DSEK procedure could safeguard against calcification. Before gas or air is used during the DSEK procedure, these authors suggest irrigating the anterior chamber and leaving it filled with balanced saline solution for at least 8 minutes—possibly while the endothelial graft is prepared—to prevent the formation of calcium phosphate crystals.20
Christophe Pagnoulle, research and development manager for PhysIOL, and Sébastien Franssens, training and product manager for the company, said that, although it may be tempting to associate a particular family of materials with risk of a certain complication, it can be a mistake to generalize. Problems may be related weaknesses in the manufacturing processes at one individual company. “This is particularly true for opacification phenomena that are sometimes reported after implantation,” Mr. Pagnoulle said. “These opacifications are of varying origins—biological (opacification of the posterior capsule), physical (water droplets producing glistening), and chemical (absorption of calcium salts)—and may, in many cases, be explained by insufficient control during polymerization and/or manufacturing processes. Certain opinions that are sometimes expounded as to the benefits or limitations of certain families of materials may thus be challenged.”
Dr. Olson noted that calcification may be seen as a more serious problem by US surgeons than by surgeons in Europe because of differences in the medicolegal climate. “While we all know the risk for calcification is greater with hydrophilic IOLs, most surgeons in Europe feel this is a minor problem, whereas in the litigious United States there is greater concern,” he said. “While this is generally true, there are hydrophilic lenses that have done very well for many years now, so this need not be a universal problem.” He added that “it is really difficult to defend in a US court a hydrophilic IOL that calcifies at this time.”
LOOKING BACK, GOING FORWARD
Advances in IOL technology may involve combining the best qualities of hydrophilic and hydrophobic materials. As mentioned previously, several manufacturers have introduced hydrophobic IOLs with higher water content. Other material advances have been investigated in preclinical work.
Researchers in Japan evaluated the biocompatibility properties of a hybrid copolymer lens with a hydrophilic center and a hydrophobic surface coating and found that it may combine some of the advantages of both types of material. When the hybrid lens was compared in vitro to hydrophobic and hydrophilic IOLs, it was less susceptible to cell adhesion than the hydrophilic IOLs and less susceptible to glistenings formation than the hydrophobic IOLs.21
Using a rabbit model, Dr. Werner and colleagues at the University of Utah investigated the biocompatibility of a one-piece hydrophobic acrylic IOL with an ultraviolet/ozone (UV-O3) coating on the posterior surface. They concluded that the treatment appeared to prevent PCO, likely by increasing adhesion between the posterior capsule and the IOL while retaining biocompatibility. There were no signs of untoward toxicity or inflammation in the rabbit eyes, the authors reported.22
“There are increasing examples of symbiotic materials coming out on the market,” Dr. Kretz said. He noted that the FineVision HP, as mentioned earlier, is a hydrophobic IOL with a higher than usual water content (4.9%). Like the company’s FineVision IOL, it is a trifocal aspheric diffractive IOL, but it is made with the company’s glistenings-free hydrophobic material rather than its hydrophilic material. “There are also hydrophobic platforms with new optical designs in trials that appear to offer great promise,” he said.
The evolution of the IOL, from rigid to flexible, has formed the backdrop of modern cataract surgery. But just as other areas of cataract surgery continue to evolve, so does IOL design.
1. Market Scope. 2018 IOL Report: A Global Market Analysis for 2017 to 2023.
2. Werner L. Material world: The growing variety of foldable IOL materials. AAO One Network. October 5,2012. https://www.aao.org/current-insight/material-world-growing-variety-of-foldable-iol-mat-3. Accessed June 19, 2018.
3. Werner L. Glistenings and surface light scattering in intraocular lenses. J Cataract Refract Surg. 2010;36(8):1398-1420.
4. Packer M, Rajan M, Ligabue E, Heiner P. Clinical properties of a novel, glistening-free, single-piece, hydrophobic acrylic IOL. Clin Ophthalmol. 2014;8:421-427.
5. Pagnoulle C, Bozukova D, Gobin L, Bertrand V, Gillet-De Pauw MC. Assessment of new-generation glistening-free hydrophobic acrylic intraocular lens material. J Cataract Refract Surg. 2012;38(7):1271-1277.
6. Werner L, Thatthamla I, Ong M, Das K, Schatz H. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison with commercially available lenses. Paper presented at: European Society of Cataract and Refractive Surgeons Annual Meeting; October 7-11, 2017; Lisbon, Portugal.
7. Heatley CJ, Spalton DJ, Kumar A, Jose R, Boyce J, Bender LE. Comparison of posterior capsule opacification rates between hydrophilic and hydrophobic single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2005;31(4):718-724.
8. Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysis. Medicine (Baltimore). 2017;96(44):e8301.
9. Li Y, Wang J, Chen Z, Tang X. Effect of hydrophobic acrylic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: meta-analysis. Plos one. 2013;8(11):e77864.
10. Ursell P, Dhariwal M, Majirska K, et al. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK Real World Evidence study [published online ahead of print June 11, 2018]. Eye (Lond).
11. Werner L, Müller M, Tetz M. Evaluating and defining the sharpness of intraocular lenses. Microedge structure of commercially available square-edged hydrophobic lenses. J Cataract Refract Surg. 2008;34:310-317.
12. Werner L, Tetz M, Feldmann I, Bücker M. Evaluating and defining the sharpness of intraocular lenses: microedge structure of commercially available square-edged hydrophilic intraocular lenses. J Cataract Refract Surg. 2009;35:556-566.
13. Werner L. Causes of intraocular lens opacification or discoloration. J Cataract Refract Surg. 2007;33(4):713-726. Review.
14. Bompastor-Ramos P, Póvoa J, Lobo C, et al. Late postoperative opacification of a hydrophilic-hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2016;42(9):1324-1331.
15. Werner L, Wilbanks G, Ollerton A, Michelson J. Localized calcification of hydrophilic acrylic intraocular lenses in association with intracameral injection of gas. J Cataract Refract Surg. 2012;38(4):720-721.
16. Werner L, Wilbanks G, Nieuwendaal CP, et al. Localized opacification of hydrophilic acrylic intraocular lenses after procedures using intracameral injection of air or gas. J Cataract Refract Surg. 2015;41(1):199-207.
17. Kim DJ, Chuck RS, Lee JK, Park CY. Reversible opacification of hydrophobic acrylic intraocular lens- two cases report. BMC Ophthalmology. 2017;17(1):111.
18. Wilkins E, Olson RJ. Glistenings with long-term follow-up of the Surgidev B20/20 polymethylmethacrylate intraocular lens. Am J Ophthalmol. 2001;132(5):783-785.
19. Hayashi K, Hirata A, Yoshida M, Yoshimura K, Hayashi H. Long-term effect of surface light scattering and glistenings of intraocular lenses on visual function. Am J Ophthalmol. 2012;154(2):240-251.
20. Lacey JC, Ghatora,BK, Foot PJS, et al. Intraocular lens calcification after DSEK: a mechanism and preventive technique. Cornea. 2016;35(9):28-30.
21. Fujita S, Tanaka T, Miyata A, Hirose M, Usui M. Cell adhesion and glistening formation in hybrid copolymer intraocular lenses. Ophthalmic Res. 2012;48(2):102-108.
22. Farukhi MA, Werner L, Kohl JC, et al. Evaluation of uveal and capsular biocompatibility of a single-piece hydrophobic acrylic lens with ultraviolet-ozone treatment on the posterior surface. J Cataract Refract Surg. 2015;41(5):1081-1087.




