How Raw Images From a Swept-Source Biometer Help My Surgical Decision-Making
Patient understanding and education also improve.
By Sumit “Sam” Garg, MD
It is commonly known that a healthy ocular surface maximizes the outcomes of IOL implantation. Just as important in predicting visual outcomes are the health and function of the macula. If a patient’s macula appears healthy, chances are that the function of the macula will also be healthy.
Obtaining retinal images preoperatively helps me to educate myself about the patient and, in turn, the patient about proper expectations for surgery. My approach begins with the desire to provide patients with an accurate estimate of how well they will see postoperatively, and it guides me in selecting the right IOL technology.
BEYOND THE OCULAR SURFACE
To identify posterior segment pathology at the outset, I have expanded my cataract workup to include imaging of the full eye. When I evaluate patients for IOL implantation, I first ask them if they had good vision before the development of their cataract. If they respond that they have never had good vision, it is a signal that I should explore other diagnoses.
Next, I ask patients to complete a cataract questionnaire that assesses how their vision affects their quality of life. The staff completes the initial workup, including performing manifest refraction, glare testing, a pinhole test for near vision (potential acuity test), ocular dominance testing, swept-source optical biometry (»IOLMaster 700, Carl Zeiss Meditec), corneal topography (»Pentacam, Oculus Optikgeräte), and IOP measurements.
My physical examination consists of examining the eye for ocular surface disease issues, such as meibomian gland dysfunction and dry eye disease. I also examine the cornea to ensure that no scarring, superficial irregularity (such as anterior basement membrane dystrophy or Salzmann nodules), or endothelial disease is present. Next, I examine the lens to determine the quality of the cataract and identify any other comorbidities that may be present. Finally, I directly examine the retina and try to identify any obvious macular or peripheral pathology.
At this point, I review the swept-source optical biometry image, which shows anatomic details of the visual pathway including the ocular surface, lens, and a single-shot OCT image of the macula. If I notice any abnormalities of the macula on biometry or in physical examination, I order a traditional OCT (»Spectralis, Heidelberg Engineering; »Cirrus, Carl Zeiss Meditec). Common OCT findings can include epiretinal membranes, macular drusen, lamellar macular holes, and edema.
For a test to be reimbursed by payers, a diagnosis is needed. Performing and analyzing the optical swept-source biometry before I order a full macular OCT helps to confirm that it is a necessary test. Other indications can also help to determine whether a traditional OCT should be ordered, for example, if the patient’s history identifies a possible issue with the macula, such as macular degeneration, diabetes, or previous retinal surgeries such as a membrane peel.
Understand that this flow is not foolproof. The single image provided by the IOLMaster 700 is not meant to be used as a screening tool for macular pathology; however, I have found that, if there are abnormalities noted on imaging provided by this device, a formal macular OCT should be considered.
If swept-source biometry imaging reveals a healthy retina and the patient is deemed to have good visual potential, I then talk to the patient about lens options, taking into account the results of the full evaluation. I ask patients if they would like to be less spectacle dependent after surgery, and whether they would tolerate glare or halos. I also ask them about their occupation and try to get a sense of their interests and hobbies. Finally, I reinforce their education with a video (Rendia) to help them learn more about their potential IOL choice, and I show them images of their macula and keratometry from swept-source biometry to help educate them further.
IS 20/20 POSSIBLE?
Every patient wants to know if he or she will be 20/20 after surgery. However, I don’t give patients a number to expect postoperatively. Like many other ophthalmologists, I compare the eye to a camera, and I explain to patients that the retina is the film, and its health is instrumental in their outcome. Even though we are replacing the lens in their camera, their film will still play a major role in the equation that determines how well they will see.
Assuming the rest of the eye is normal, I tell patients, if there is an irregularity in the retina, it may worsen over time and therefore affect their visual outcome. I am careful to make sure to explain to patients why the status of the retina affects lens choice. I generally prefer a conservative approach in these patients. I favor monofocal or toric IOLs that are not affected by the macular health as a presbyopia-correcting IOL would be. In eyes with significant macular pathology or with pathology that is likely to progress with time, I generally avoid multifocal or extended depth of focus IOLs and counsel these patients about the effect of their macular pathology on their expected visual outcome.
CASE EXAMPLES
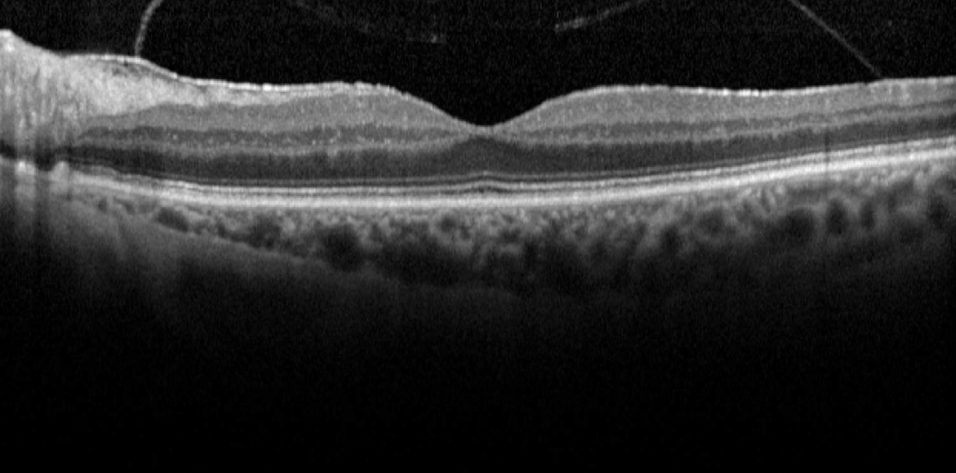
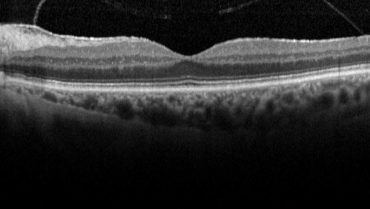
Case No. 1. A 74-year-old woman presented with a moderately symptomatic cataract. Her BCVA in each eye was 20/40. Swept-source biometry (Figure 1) showed good keratometry and a good macular contour, and macular OCT (Figure 2) showed a normal foveal contour. This demonstrates how a normal swept-source single-shot OCT correlates with a normal full macular OCT.
Figure 1. Swept-source biometry for this patient showed good keratometry and a good macular contour.
(Images courtesy of Sumit “Sam” Garg, MD)
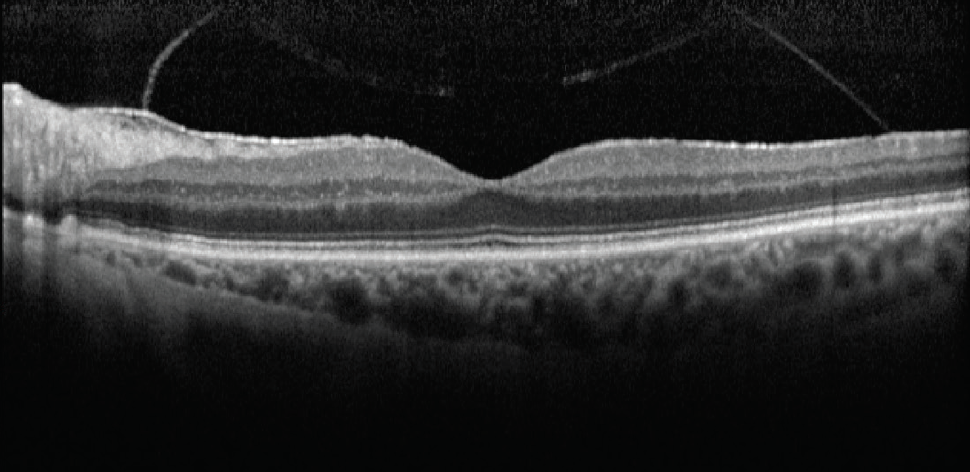
Figure 2. Macular OCT of same eye in Figure 1 showed a normal foveal contour.
(Images courtesy of Sumit “Sam” Garg, MD)
Case No. 2. A 66-year-old man came to the office complaining of trouble driving at night. He had been told that he had fluid in his eye but was unsure which eye. His BCVA was 20/50 OD and 20/40 OS. Swept-source biometry showed a cystic appearance on the single-shot OCT of his right eye and a normal appearing macula in his left (Figure 3). Macular OCT confirmed cystoid macular edema (CME) in his right eye. The left OCT macula was within normal limits (Figure 4). Because I believed the patient had a mechanical component to his CME from an epiretinal membrane, he was sent for evaluation by a retinal subspecialist. On consultation, it was decided to perform a combined retinal membrane peel and cataract surgery in the patient’s right eye. He had an irregular keratometry in his right eye (Figure 3). On slit-lamp examination, he had Salzmann nodules on his right cornea. I plan to proceed with a superficial keratectomy in his right eye and repeat swept-source biometry to better target his IOL power.
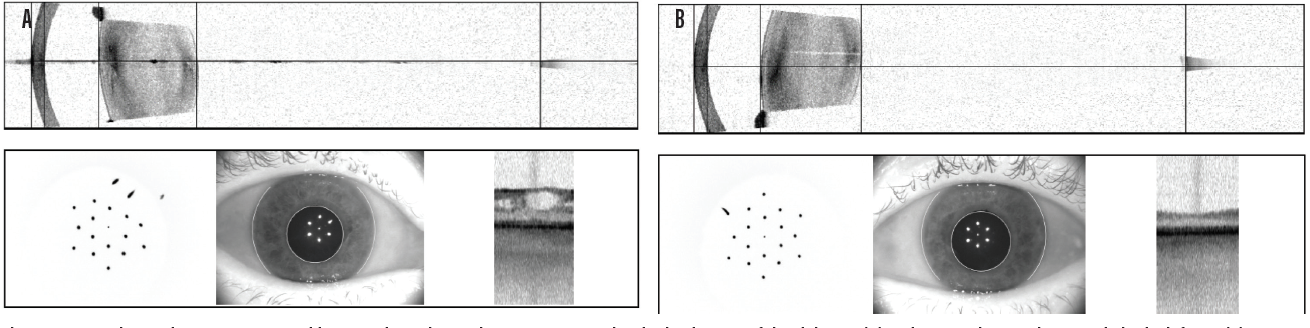
Figure 3. In another patient, swept-source biometry showed a cystic appearance on the single-shot OCT of the right eye (A) and a normal appearing macula in the left eye (B).
(Image courtesy of Sumit “Sam” Garg, MD)
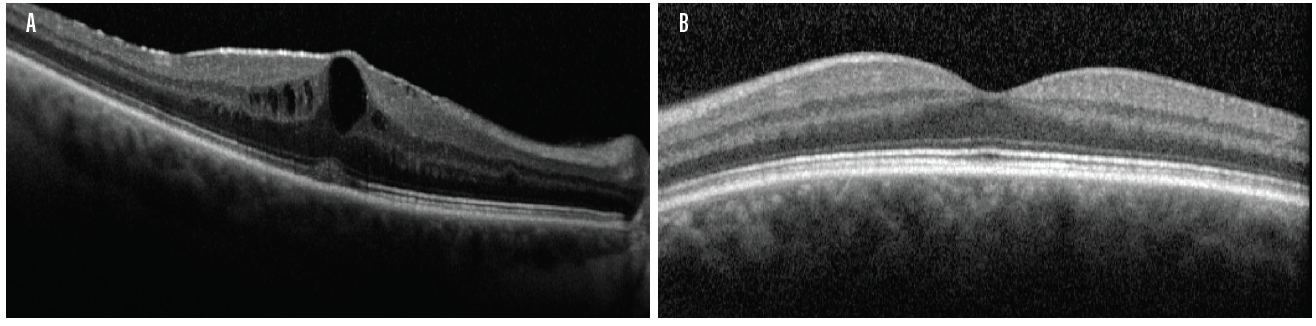
Figure 4. Macular OCT of the same patient in Figure 3 confirmed CME in the right eye (A). The left OCT macula was within normal limits (B).
(Image courtesy of Sumit “Sam” Garg, MD)
CONCLUSION
Technologies that give me a sense of the health of the retina can be a springboard for more in-depth testing when necessary. There is a big focus in ophthalmology on ocular surface disease and maximizing the ocular surface, but it is equally important to ensure that the retina is healthy and disease-free. Treating dry eye but overlooking an epiretinal membrane affects visual quality just as much as treating the epiretinal membrane and overlooking the dry eye.
Keep in mind that the IOLMaster 700 is not designed to be a screening tool for macular disease or dry eye disease. That being said, I have found that the images it provides are useful tools to help me decide if further testing is warranted, and they also can be used to aid the discussion with patients regarding their options.
As the adage goes, if we diagnose it before surgery, it’s the patient’s problem; if we diagnose it after surgery, it is our problem.

Taking Stock of What We Cannot See
Evaluating the retina under dense cataracts can be challenging, but it can tell us if the risks of surgery are warranted.
By Mitchell A. Jackson, MD
Managing patients with dense cataracts or other media opacities can be tricky. In cataract surgery, nucleus fragmentation with a femtosecond laser has been shown to reduce effective phacoemulsification time,1 but surgical times are often longer in patients with brunescent cataracts, and the potential for iatrogenic trauma is increased. The risk of complications, including lens subluxation and posterior capsular tears, is also higher with brunescent cataracts than with less dense cataracts.2
These types of cataracts, as well as other media opacities, therefore present unique challenges for preoperative planning. It can be difficult to determine the prognosis for postoperative visual recovery when the view of the macula is limited. Imaging modalities such as OCT and fundus photography are also unreliable in the presence of dense media opacity.
B-scan ultrasound can uncover some hidden posterior segment pathologies in these cases, but ultrasound does not convey any information about the health of the retina. Therefore, it has a limited role for diagnostic purposes, especially when there is suspicion of retinal pathology.
In cases such as these, visual electrophysiology testing with electroretinography (ERG), in particular flicker ERG (fERG), can provide objective information about global retinal function in patients being evaluated for cataract surgery.
WHAT IS FERG?
In fERG, as performed with the »Diopsys ERG (Diopsys), alternating flashes of dark and light stimuli at 32 Hz are used to stimulate the retina, and the response is measured by a sensor placed on the patient’s lower eyelid. Rods do not respond to stimuli faster than 30 Hz, so this test provides a good evaluation of cone response. The magnitude of the signal indicates the strength of the response, and the phase indicates the timing of the response.
In simple terms, like visual field testing, fERG provides the clinician with objective information about the function of the retina. Serial OCT provides information about structure in retinal pathologies, but it says nothing about function. Serial fERG can demonstrate deterioration over time or improvement in response to treatment. One study showed that fERG signals improved after treatment with anti-VEGF agents in patients with diabetic macular edema, suggesting that clinicians may be able to adjust therapy based on retinal function response.3 In preoperative cataract evaluation, fERG can help to determine the presence of retinal dysfunction that might be otherwise undetectable behind a dense opacity.4,5
CLINICAL DECISION-MAKING
In planning cataract surgery, it is vital to understand when surgery might involve more risk than benefit. One of the hardest things surgeons have to do is to tell a patient that he or she is not a good candidate for a cataract operation—that the surgery might do more harm than good. For instance, brunescent cataracts are likely to be encountered in older patients who are also likely to have age-related systemic diseases or ocular pathologies, and these factors may heighten the risks of surgery.
I recall seeing a patient who used a wheelchair and had opaque cataracts in each eye that provided no view of the fundus. She came to my clinic for a cataract surgery consultation. Due to her systemic health issues, I was doubtful of the prospect for her postoperative recovery and her potential for visual gain. Testing with fERG indicated highly abnormal retinal function, confirming my suspicion: With the patient’s ill health combined with little or no chance of visual recovery, I might do more overall harm by subjecting her to surgery to remove the cataract. It was certainly not an easy conversation to have with the patient and her family, but it was also my obligation to inform her that the risks for a dropped lens, increased inflammation, and potentially little to no visual improvement outweighed the benefits of the operation.
On the other hand, fERG can help to determine when cataract surgery is likely to be beneficial in doubtful cases. I had a consultation with a 78-year-old woman with brunescent cataracts in each eye. Her UCVA was count fingers at 3 feet OD and 20/200 OS. BCVA was count fingers at 3 feet OD and 20/100 OS. During preoperative evaluation, it was not possible to examine the fundus in the patient’s right eye, and view was limited in her left. OCT imaging was of little value for assessing structure (Figure 5) and no value for assessing function. Testing with fERG, however, indicated a normally functioning retina (Figure 6), suggesting a good prognosis for visual recovery.
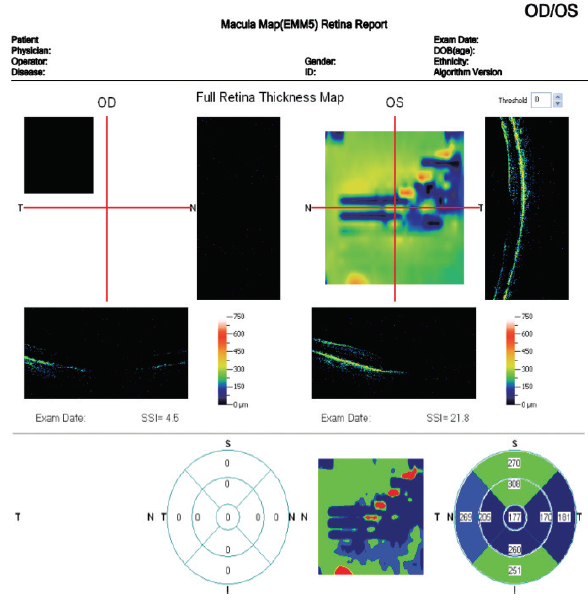
Figure 5. Preoperative evaluation of a 78-year-old woman was complicated by a dense media opacity, which limited the usefulness of OCT.
(Images courtesy of Mitchell A. Jackson, MD)
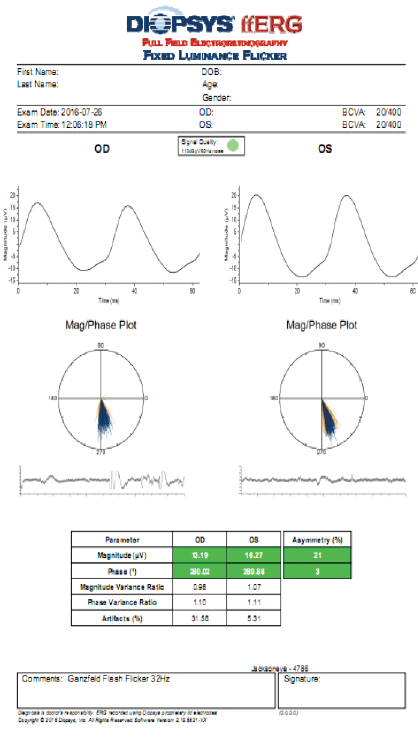
Figure 6. Testing with fERG provided information about retinal function that confirmed the decision to perform cataract surgery with IOL placement.
(Images courtesy of Mitchell A. Jackson, MD)
In this instance, fERG helped confirm the decision to perform cataract surgery. Postoperatively, the patient’s BCVA improved to 20/40 OD and 20/20 OS. A postoperative OCT image showed a fully intact and healthy retina (Figure 7).
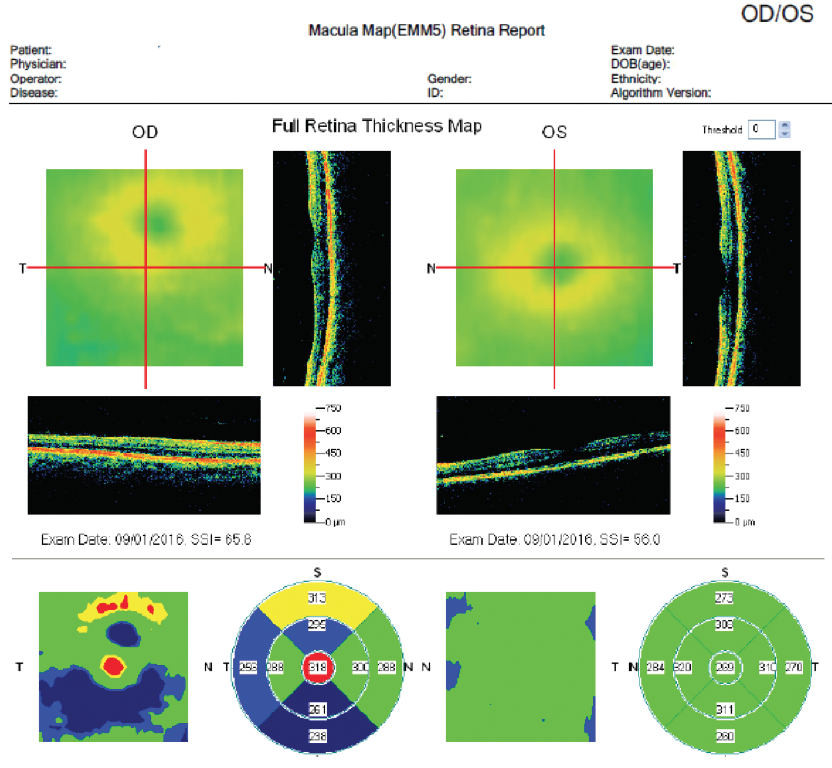
Figure 7. Postoperative OCT of same patient in Figure 5.
(Images courtesy of Mitchell A. Jackson, MD)
Performing fERG in patients with dense cataract can help establish and set patient expectations for postoperative vision. In patients with bilateral cataracts, if fERG demonstrates greater abnormality in one eye, I can use that information to properly educate the patient on potential outcomes. Explaining the prognosis is made simpler when I can show patients an objective measurement with color-coded results.
We do not bill for fERG unless there is an abnormal test result. We use it mainly for screening purposes, to aid in decision-making in patients with a dense cataract, and we also use it as an additional aid to determine if a patient with suspected macular pathology such as an epiretinal membrane or mild age-related macular degeneration is a good candidate for a presbyopia-correcting IOL.
CONCLUSION
Most high-volume cataract surgeons will encounter cataracts that limit or prohibit the view of the fundus fairly regularly. The risk-benefit analysis in these eyes is greatly facilitated by the availability of objective, quantifiable data about the function of the retina, particularly the cone cells. In my clinic, fERG is a routine part of evaluating such cases for planning surgery and managing expectations.
Another way fERG can be used in modern cataract surgery is to help determine if the retina is healthy enough to support implantation of a multifocal IOL and to counsel patients about these premium lenses. My preference is to avoid premium lenses if there is any hint of retinal pathology. That being said, fERG testing can help confirm whether a patient is a good candidate for a premium IOL, thereby lessening the chance for an unhappy patient disappointed with the postoperative visual result.
Objective, quantifiable data is our greatest ally in assessing challenging cases. As surgeons, we tend to trust our instincts and training, but it is hard to do that when our view is limited. Taking stock of the health of the retina in the presence of dense cataracts with fERG—even if we cannot see it clearly—can help confirm our decisions on whether and how to proceed with cataract surgery.

Wide Retinal View Improves Assessments, Efficiency, Engagement
Ultra-widefield imaging can benefit both surgeon and patient.
By Steven G. Safran, MD
Assessing retinal health is an essential element of both pre- and postoperative responsibilities for the cataract surgeon. A thorough evaluation can assist in the selection of IOLs and can help the surgeon uncover pathology that could affect surgical outcomes or lead to potential postoperative complications. Despite these benefits, surgeons can face challenges in incorporating this crucial step into practice protocol, including efficiency considerations and problems with patients who either don’t dilate well or refuse to be dilated.
Eighteen months ago, I acquired the »California ultra-widefield (UWF) imaging device (Optos). The device’s optomap nonmydriatic UWF technology delivers detailed 200° images (82% of the retinal area) in a single capture, providing a global view of the retina in less than 0.5 seconds. In combination with OCT, employing this retinal roadmap in my pre- and postoperative evaluations helps me to ensure that I have an excellent view of the macula and the peripheral retina, and it gives me additional information that I might have missed using an indirect ophthalmoscope.
PREOPERATIVE INTELLIGENCE
When conducting a preoperative evaluation, I first want to confirm that cataract is the only issue affecting the patient’s vision. Using the high-resolution optomap image to carefully examine the central and peripheral retina allows me to identify pathology, determine if it must be addressed prior to surgery, and assess surgical risks. This is particularly important for patients who either do not dilate well or are at elevated risk for retinal issues, including those with high myopia, diabetes, or previous retinal procedures, and those presenting for lens exchange requiring vitrectomy.
Using UWF imaging, I have identified and subsequently treated or referred a host of pathologies including peripheral holes, tears, detachments, tumors, and vascular abnormalities that might otherwise have taken a greater amount of time to uncover or, worse, been missed entirely. UWF imaging has also allowed me to recognize previous retinal surgeries in patients who either did not have complete medical records or did not disclose this as part of their history.
In patients who do not dilate well or refuse to be dilated, an optomap image can serve as reassurance of the absence of pathology or as a tool for convincing them to be dilated so that an identified issue can be further evaluated and ultimately treated. The images can also serve as documentation of preexisting conditions, which is particularly important for patients who are at greater risk for complications.
The information garnered from the expansive UWF peripheral view can also help guide the surgeon’s IOL recommendations and allow greater understanding of the patient’s potential postoperative outcomes. This can help to manage the expectations of both the surgeon and patient and maximize chances for surgical success and satisfaction.
POSTOPERATIVE EVALUATION, PATIENT ENGAGEMENT
UWF imaging can be of equal if not greater value in the postoperative period. Patient reports of flickering, flashes, or floaters after surgery must be taken seriously, but dilating every case immediately may be unnecessary. Using the optomap device to image these patients allows me to evaluate practically the entire retina to determine what may be causing the issue and decide what, if any, further action is required. These images can also serve as documentation of postsurgical outcomes.
In addition to being valuable diagnostic and assessment tools, optomap images offer a unique way to engage with patients. There is no more impactful way to show and explain certain retinal pathologies. I find that sharing before and after images with patients helps them to better understand their diagnosis preoperatively and to feel gratified when they see treatment results postoperatively (Figure 8).
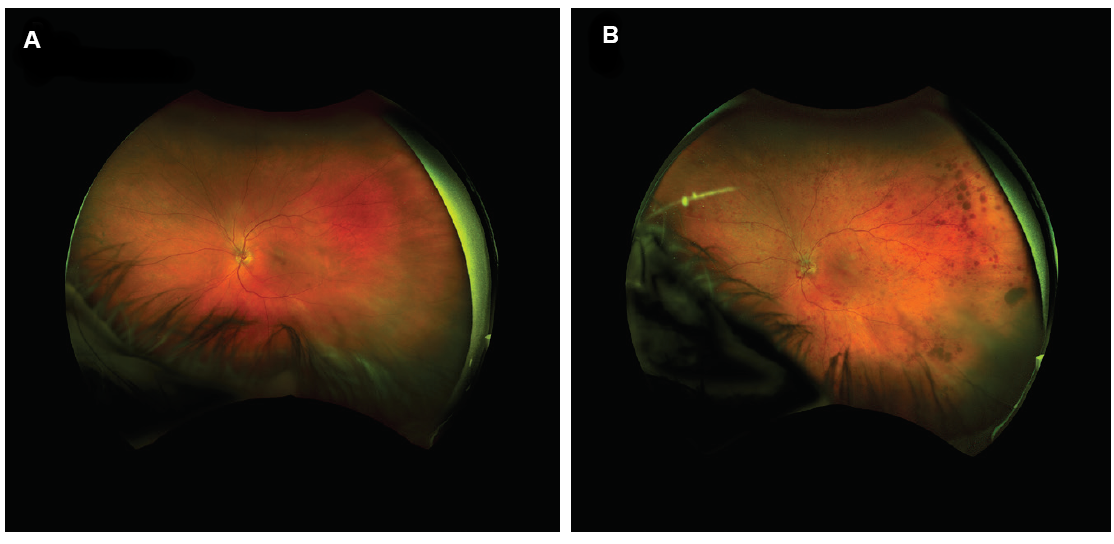
Figure 8. UWF image of patient’s eye in 2017 (A); the same eye of the same patient in 2018 with central retinal vein occlusion (B).
(Images courtesy of Steven G. Safran, MD)
Recently, I saw a patient—a thoracic surgeon—who was seeking a third opinion. He had been told by one ophthalmologist that he had anterior ischemic optic neuropathy, and a second had diagnosed a vein occlusion. The optomap image revealed signs of a vein occlusion in the periphery. When I shared the image with the patient, he was able to see and understand my diagnosis.
In another patient, for whom I performed a lens exchange and pars plana vitrectomy, superficial bleeding occurred when I extracted the trocar, leaving behind a few small drops of blood in the vitreous. I used an optomap image to explain the situation and assure him that it would resolve, which helped to allay his concerns.
PRACTICE EFFICIENCY
Regardless of the clinical benefits of any new technology, its impact on practice efficiency must also be considered. As the only surgeon in my practice, I often need my staff to assist wherever appropriate. The Optos device is easy to learn and to operate. Every member of my staff knows how to capture and recognize a high-quality, reproducible image. They also know to take an optomap image upon patient arrival in any situation in which it might be valuable. This can include patients complaining of flashes or floaters, highly myopic patients, and those who have had previous retinal surgeries.
The staff is also trained to target peripheral pathology or abnormalities on which I need to focus. For example, for a patient with a displaced lens sitting on the retina inferiorly, they know to have the patient look down to capture the lens in the image. In the examination room, staff members can pull up images on the monitor so that I can review them with the patient. Reviewing images with staff members present has had the added benefit of giving them a greater understanding of what to look for in the future; this has increased staff engagement and made them better at what they do.
CONCLUSION
Having access to a 200° view of the retina has allowed me to identify and address pathologies that I almost certainly would have missed without it. This, combined with the positive impact UWF has had on practice efficiency and patient engagement, has allowed me to deliver a higher level of care.
1. Hatch KM, Schultz T, Talamo JH, Dick HB. Femtosecond laser-assisted compared with standard cataract surgery for removal of advanced cataracts. J Cataract Refract Surg. 2015;41(9):1833-1838.
2. Basic and Clinical Science Course. Section 11: Lens and Cataract. San Francisco: American Academy of Ophthalmology; 2014-2015: 39-41.
3. Holm K, Schroeder M, Lövestam Adrian M. Peripheral retinal function assessed with 30-Hz flicker seems to improve after treatment with Lucentis in patients with diabetic macular oedema. Doc Ophthalmol.2015;131(1):43-51.
4. Foerster MH, Li XX. Evaluation of the central retina and optic nerve function in media opacities. Doc Ophthalmol. 1986;63(1):101-106.
5. Ratanapakorn T, Patarakittam T, Sinawat S, et al. Effect of cataract on electroretinographic response. J Med Assoc Thai. 2010;93:1196-1199.