It has been a long road. After 65 years of iris prostheses, the United States has finally seen its first approved device in this category.
The first iris prostheses were implanted by D. Peter Choyce, FRCS, in the United Kingdom in the 1950s. After a short initial run, the field was quiet for nearly 4 decades, until the early 1990s when interest was rekindled in Europe and a few manufacturers began making devices. Despite the demonstrated clinical usefulness of these products elsewhere in the world, in the United States, prior to the May 2018 approval of the CustomFlex artificial iris (HumanOptics), use of artificial irides had been limited to a relatively small number of patients, either within FDA investigational device exemption (IDE) trials or under compassionate use device exemptions. Those restrictions are now about to change, as practitioners can begin to use the CustomFlex artificial iris to treat patients with congenital aniridia and iris defects resulting from other conditions such as albinism, trauma, or surgical iris removal.
Investigators in the FDA IDE trial for the CustomFlex (see Who’s Who) have been pleased with the performance of the device.
Who’s Who
CustomFlex FDA Study Investigators and Regulatory Team Members
- Brandon D. Ayers, MD
- Robert J. Cionni, MD
- Alan S. Crandall, MD
- Barbara Fant, PharmD
- Nicole R. Fram, MD
- Marshall Bowes Hamil, MD
- Stephen M. Hamilton, MD
- David Hardten, MD
- Richard S. Hoffman, MD
- Douglas D. Koch, MD
- Richard J. MacKool, MD
- Samuel Masket, MD
- Kevin M. Miller, MD
- Francis W. Price Jr, MD
- Jodi Pitcher
- Irving M. Raber, MD
- Kenneth J. Rosenthal, MD
- Michael E. Snyder, MD
- R. Doyle Stulting, MD
CUSTOM MEANS CUSTOM
Unlike any other ophthalmic implantable device, each CustomFlex unit is manufactured for a specific patient (Figure 1). For patients who are affected monocularly, whether due to trauma, iridocorneal endothelial syndrome, or the like, the custom prosthesis is manufactured based on a photograph taken of the unaffected fellow eye. There is a process for getting the photo lighting and color just right.
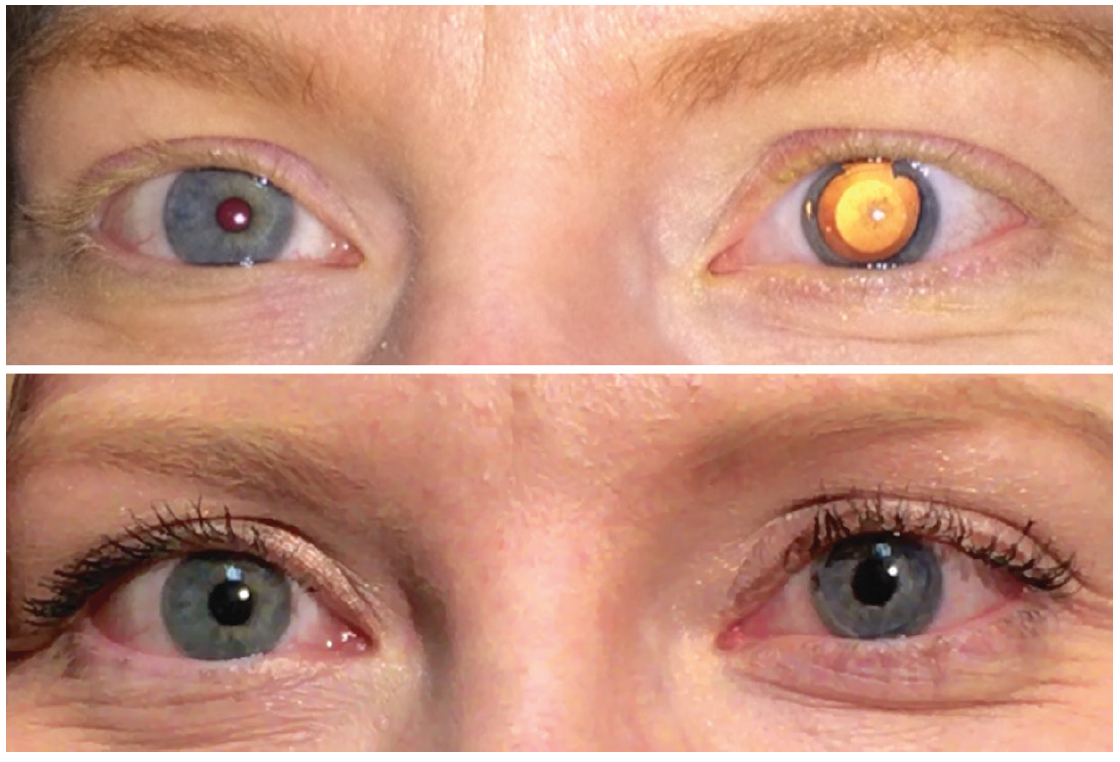
Figure 1. A patient with iris damage after phakic IOL implantation, before (top) and after (bottom) CustomFlex implantation.
Patients with congenital aniridia have the opportunity to identify a hard copy photograph of any eye they find appealing. Their devices are then handcrafted based on the index photo, polymerized, sterilized, and sent to the surgeon. There are no off-the-shelf options or the just-in-time delivery that we have come to expect from Amazon.com orders. The duration from decision to implantation can be a few months, requiring patience from the patient and the doctor.
These devices are designed and intended only for use in the treatment of pathologically affected eyes, and they do a great job of returning a diseased or affected eye to a more normal appearance. They are neither intended nor advised for the purpose of eye color change. Please do not confuse these carefully tested, meticulously manufactured, customized, flexible artificial irises with other anterior chamber products that are implanted strictly for iris color change. Those units have not been tested in IDE studies and have left a trail of peer-reviewed case series describing sight-threatening complications. In contradistinction, the customized, flexible CustomFlex has been tested in the FDA clinical trial, and its manufacturing process has been evaluated under the rigors of FDA regulations.
BLACK DEVICES
In some cases, patients’ pathologies may be limited to loss or absence of the iris pigment epithelium, such as ocular or oculocutaneous albinism, eyes that have exhibited intraoperative floppy iris syndrome, or uveitic or idiopathic iris pigment atrophy. For patients like these, the device can be made in black, although they are still custom-manufactured on a case-by-case basis.
Albinotic eyes, which often appear pink in their native state, tend to appear a vivid blue (think Paul Newman) after a black device has been placed behind the unpigmented stroma (Figure 2).
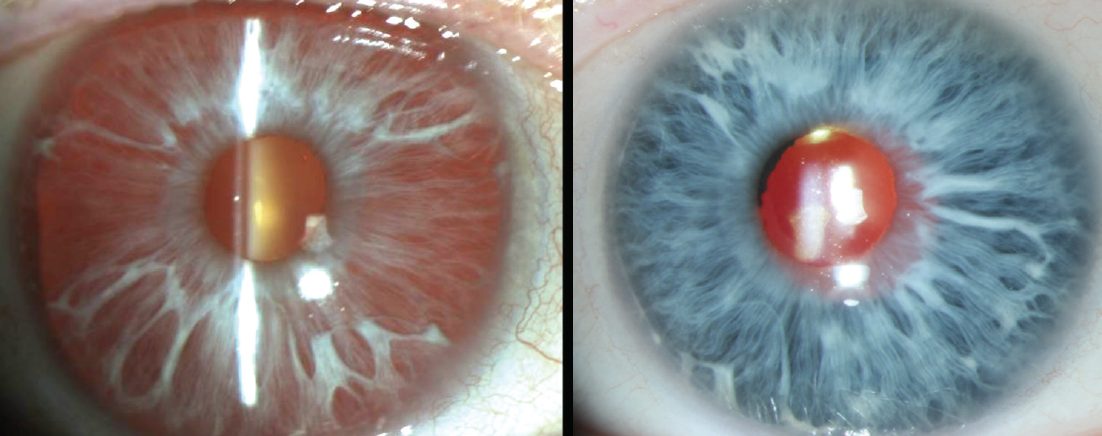
Figure 2. An albinotic iris before (left) and after (right) implantation of a black CustomFlex.
SKILLS NEEDED
As with any new technology or procedure, surgeons must consider their practice mix, training, and experience when incorporating a new skill into their practices. Eyes suited to iris prosthesis placement typically come with significant comorbid pathologies, so surgeons tackling such cases should have experience with eyes of similar complexity and should have the skills needed to fixate an IOL without capsular support and to perform suturing within the anterior segment. These types of manipulations are often required in eyes needing iris prostheses, and a similar level of dexterity is involved in implantation of the device.
Now that the CustomFlex is approved in the United States, HumanOptics plans to initiate a robust surgeon training program.