Conservative Techniques Are Appropriate to Start
Patients should be counseled that the state of their corneas may compromise the outcome.
CRST: How often do patients with a history of radial keratotomy (RK) surgery present to your practice?

Jorge L. Alió, MD, PhD, FEBO: I see these patients regularly. RK was popular in my region of Spain, and I often performed the procedure 30 years ago.
CRST: How do you manage patients who experience a progressive hyperopic shift after RK?
Professor Alió: I start conservatively by prescribing glasses because the refraction is typically unstable.
CRST: How do you manage patients who have developed ectasia after RK?
Professor Alió: None of the patients I have seen who had healthy corneas when they underwent RK has developed ectasia. Others, however, had keratoconus when they underwent RK by another surgeon—many of those who performed RK when the procedure was popular did not have a corneal topographer to detect the disease. My experience treating these patients is poor. If CXL does not work well, then I suspect corneal topography was not performed before the RK procedure.
CRST: Would you consider a phakic IOL for an RK patient with continuous hyperopia? If so, please describe your approach and/or share an example.
Professor Alió: I consider phakic IOL implantation if the patient demonstrates refractive stability for at least 18 months. I favor a scleral approach so as not to destabilize the cornea further. I prefer phakic IOL implantation to refractive lens exchange (RLE) because the refraction may not remain stable during the next few years. It is important to explain to patients that the state of their corneas may compromise the postoperative result.
CRST: How often do you see patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, and what are some of their common complaints?
Professor Alió: I see them frequently. I began performing LASIK in 1991. Many patients who had LASIK in its early days remain satisfied with their vision because their brain has adapted to any spherical aberration they have.
Wavefront analysis is performed. If the patient is happy with their vision, no other steps are taken. If not, they are offered cataract surgery with either a spherical or an extended depth of focus (EDOF) IOL that has high positive spherical aberration such as a RayOne EMV (Rayner). I find these patients do well.
CRST: Would you implant a multifocal IOL in a patient who has a history of RK or early LASIK?
Professor Alió: No, I favor monofocal lenses with positive or negative spherical aberration to compensate for the corneal optics. In select cases, I also consider an EDOF IOL such as the Tecnis Eyhance (Johnson & Johnson Vision).
CRST: Would you implant a small-aperture IOL in a patient who has a history of RK or early LASIK?
Professor Alió: My experience with the IC-8 Apthera (Bausch + Lomb) is not as good as the experiences reported by other surgeons. The patients in whom I implanted the lens have not done well, so I have stopped offering the procedure until further reliable evidence is available. Additionally, the large incision required to implant these lenses can further destabilize a cornea already weakened by RK.
CRST: Would you implant a Light Adjustable Lens (LAL; RxSight) in a patient who has a history of RK or early LASIK?
Professor Alió: I would not because my recent personal data suggest the lens is imprecise (unpublished).
CRST: What is your preferred IOL power calculation for patients who underwent RK or early LASIK?
Professor Alió: I rely on the postrefractive surgery calculators offered by the ASCRS and ESCRS and average the results of the most reliable formulas, which for me tend to be the Barrett True K and Haigis.
CRST: If you use intraoperative aberrometry, what specific benefits does the technology offer for patients who underwent RK or early LASIK?
Professor Alió: I stopped using intraoperative aberrometry because I did not find it improved outcomes.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK or early LASIK?
Professor Alió: IOP readings are often imprecise in these eyes. Conversion formulas using data obtained with the Ocular Response Analyzer (Reichert Technologies) are used to improve accuracy. In this population, I largely rely on my experience and observations, and I monitor the results of visual field and, especially, optic nerve fiber analysis in eyes with less than 6.00 D of myopia before RK.
CRST: What tips do you have for treating ocular surface disease (OSD) and dry eye disease (DED) in eyes that underwent RK or early LASIK?
Professor Alió: Patients are evaluated with the Keratograph (Oculus), and they receive eye drops in accordance with the type of disease they are experiencing. My approach to these patients is the same as for individuals with virgin corneas.
Overcoming Refractive Instability and Hyperopic Shift
Patients who underwent refractive surgery 20+ years ago have long since adapted to their higher-order aberrations.
CRST: How often do patients with a history of RK surgery present to your practice, and what are some of the common complaints, complications, and challenges you face when treating these patients?

Jorge L. Alió del Barrio, MD, PhD, FEBOS-CR: Every month, I see some patients who have a history of RK. Of greatest concern are the refractive instability and hyperopic shift these patients experience, which can render treatment with refractive surgery complex.
CRST: How do you manage patients who experience a progressive hyperopic shift after RK?
Professor Alió: In the absence of cataract, I mainly prescribe glasses because, in this situation, laser refractive enhancements are complex and unreliable and they have low predictability. For some patients, phakic IOLs may be an option.
CRST: How do you manage patients who have developed ectasia after RK?
Professor Alió: CXL may be an option, but its efficacy in this situation has not been established. For vision correction, I mainly use rigid gas permeable contact lenses. Epikeratoplasty with Bowman layer transplantation is an interesting alternative in select patients.
CRST: How do you manage patients who have corneal scars or Salzmann nodular degeneration that requires surgery after RK?
Professor Alió: I find superficial keratectomy to be a reliable method that is easy to perform and generally successful.
CRST: Would you consider a phakic IOL for an RK patient with continuous hyperopia? If so, please describe your approach and/or share an example.
Professor Alió: A phakic IOL is usually an option because these individuals generally had high myopia before undergoing RK. My preference is an EVO ICL (STAAR Surgical). The Artiflex (Ophtec) is not an option for hyperopia, and the Artisan (Ophtec) requires a large scleral tunnel for implantation.
CRST: Would you consider RLE in a patient with a history of RK?
Professor Alió: I would not consider this procedure if the patient is younger than 55 years of age owing to the retinal risks. If there is no cataract, my first choice of intervention would be a phakic IOL. If a cataract is present and the patient is at least 55 years old, I would consider RLE with a monofocal, toric, or small-aperture lens—not an EDOF or multifocal IOL.
Case Example
A patient with a history of radial keratotomy presented for a refractive surgery evaluation. Superficial scarring was visible at the slit lamp (Figure 1). Anterior segment OCT, including high-resolution imaging to display the anamorphic cornea, was performed with the MS-39 (CSO). Imaging showed the central scar was intraepithelial without involving the underlying stroma (Figure 2). A superficial keratectomy was planned and executed successfully. The opacity was fully removed, and visual function was restored.
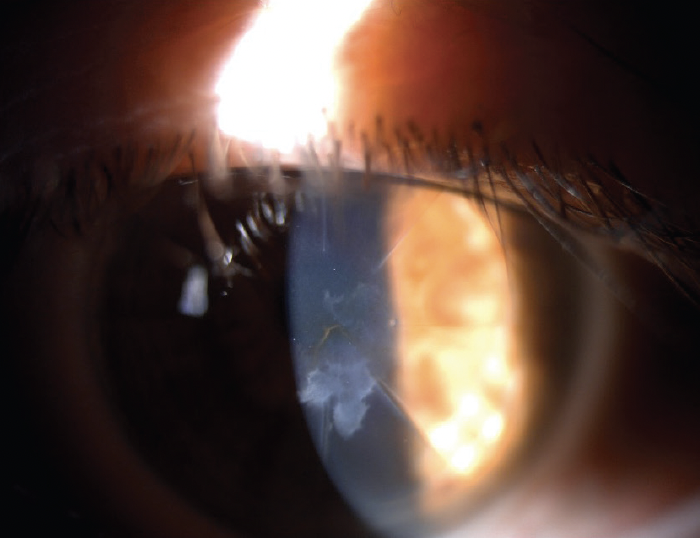
Figure 1. The eye at the slit lamp.
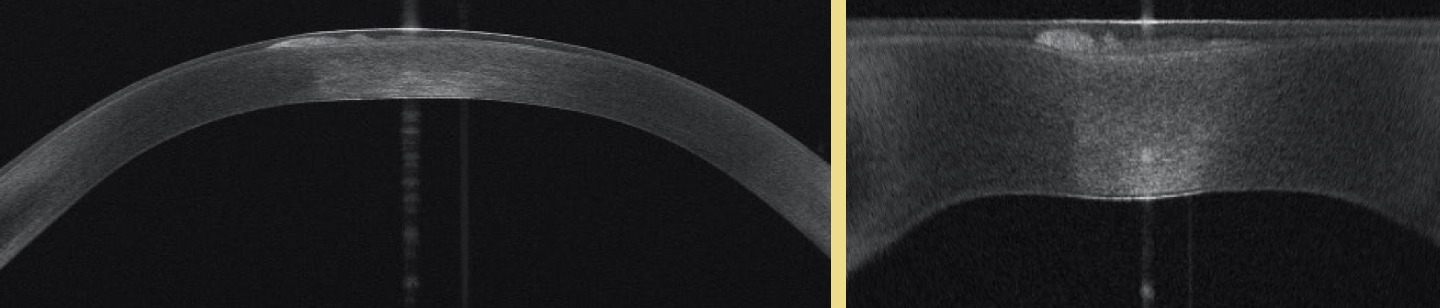
Figure 2. Anterior segment OCT images showing the central scar.
CRST: How often do you see patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, and what are some of their common complaints?
Professor Alió: I see them frequently. Common complaints are presbyopia and myopic progression.
CRST: Is an additional workup required in these patients, for example, to detect small, irregular, or decentered ablations; higher-order aberrations (HOAs); or epithelial basement membrane dystrophy?
Professor Alió: These patients have HOAs starting the day after surgery. If they did not complain about it then, they will not now. They are used to their postoperative visual acuity and vision quality. Instead, they present to an ophthalmologist when they begin to experience an increase in myopia or develop presbyopia. I offer a refractive enhancement with a flap lift, if possible, or PRK over the flap if not.1 I offer a phakic IOL only in extreme situations.
CRST: Would you implant a multifocal IOL in patient who has a history of RK or early LASIK?
Professor Alió: I would not for a patient who underwent RK. For someone who underwent an early LASIK procedure, however, the decision would depend on the results of preoperative topography and aberrometry. I am generally willing to implant a multifocal IOL only in a post-LASIK eye with a low amount of HOAs. I favor an EDOF IOL and a monofocal IOL for a post-LASIK eye with a moderate or high amount of HOAs, respectively.
CRST: Would you implant a small-aperture IOL in a patient who has a history of RK or early LASIK?
Professor Alió: I would for an a post-RK eye but not for a post-LASIK eye unless the amount of HOAs was very high. This, however, is an uncommon scenario.
CRST: Would you implant an LAL in a patient who has a history of RK or early LASIK?
Professor Alió: I do not have experience with this technology.
CRST: What is your preferred IOL power calculation for patients who underwent RK or early LASIK?
Professor Alió: I use the postrefractive IOL calculator from the ASCRS in these situations.
CRST: If you use intraoperative aberrometry, what specific benefits does the technology offer for patients who underwent RK or early LASIK?
Professor Alió: I do not have access to or experience with this technology.
CRST: Do you consider a two-step procedure with a laser ablation followed by cataract surgery for individuals who have irregular corneal astigmatism as a result of their previous refractive procedure?
Professor Alió: This could be a reasonable approach, but as I said earlier, patients usually do not seek solutions to HOAs 20 years after surgery. They have adapted.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK or early LASIK?
Professor Alió: I would adjust IOP measurements to account for corneal thickness.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK or early LASIK?
Professor Alió: My approach would be the same as in other patients and would depend on disease severity.
1. Alió Del Barrio JL, Hanna R, Canto-Cerdan M, Vega-Estrada A, Alió JL. Laser flap enhancement 5 to 9 years and 10 or more years after laser in situ keratomileusis: safety and efficacy. J Cataract Refract Surg. 2019;45(10):1463-1469.
When and How to Intervene
CRST: How often do patients with a history of RK surgery present to your practice, and what are some of the common complaints, complications, and challenges you face when treating these patients?

Bret L. Fisher, MD: Seeing patients who have had RK is a common occurrence in my practice. Several hundred thousand RK procedures were performed in the United States in the latter part of the 20th century, making it the most common refractive procedure performed during that time.1 Approximately 10% of US ophthalmologists were trained in RK and performed the procedure.
Patients with a history of RK present to my office regularly. A growing number of them are developing age-related cataracts and seeking treatment. Many, including those who received an initial monovision correction with RK and those who remained relatively spectacle independent until the onset of their cataracts, wish to be as free of spectacles as possible. Others have experienced a change in their refractive state and wish to regain clear vision without the need for glasses or contact lenses.
Patients who underwent RK can experience exaggerated glare and photic phenomena from their existing corneal scars and their developing lens opacity. Hyperopia and astigmatism, both regular and irregular, are commonly found on examination. Some patients have a degree of refractive instability and experience a progressive myopic shift over the day as the cornea steepens.
Cornea- and lens-based treatment options are available for patients who developed progressive hyperopia after RK. Surgeons (including me) have successfully performed both PRK and LASIK on individuals with a history of RK who have otherwise clear media. Patients with high hyperopia, dysfunctional lens syndrome, or lens opacity, however, are better served by newer lens-based surgical options. In the United States, a lens-based surgical option entails a lens exchange for individuals with hyperopia or hyperopic astigmatism because phakic IOLs are not yet available for these refractive errors. Choosing where on the spectrum of crystalline lens dysfunction and opacification to intervene is dictated by the patient’s level of visual disability, their ability to use other refractive options such as glasses or contact lenses successfully, and their desires after a thorough and well-documented discussion with their ophthalmologist.
Case Example
A 69-year-old woman reported blurry vision, starbursts, halos, and increasing difficulty performing the activities of daily living. She had undergone bilateral radial keratotomy for myopia in the distant past and bilateral LASIK years later. She said that she had experienced some starbursts, halos, and monocular diplopia since undergoing radial keratotomy but stated that her vision had deteriorated further during the past few years.
On examination, the patient’s BCVA was +2.00 -0.75 x 095º = 20/50-1 OD and +2.75 -1.25 x 066º = 20/40 OS. A slit-lamp examination found moderate nuclear sclerosis and cortical changes in each eye. The remainder of the examination was unremarkable (Figure).
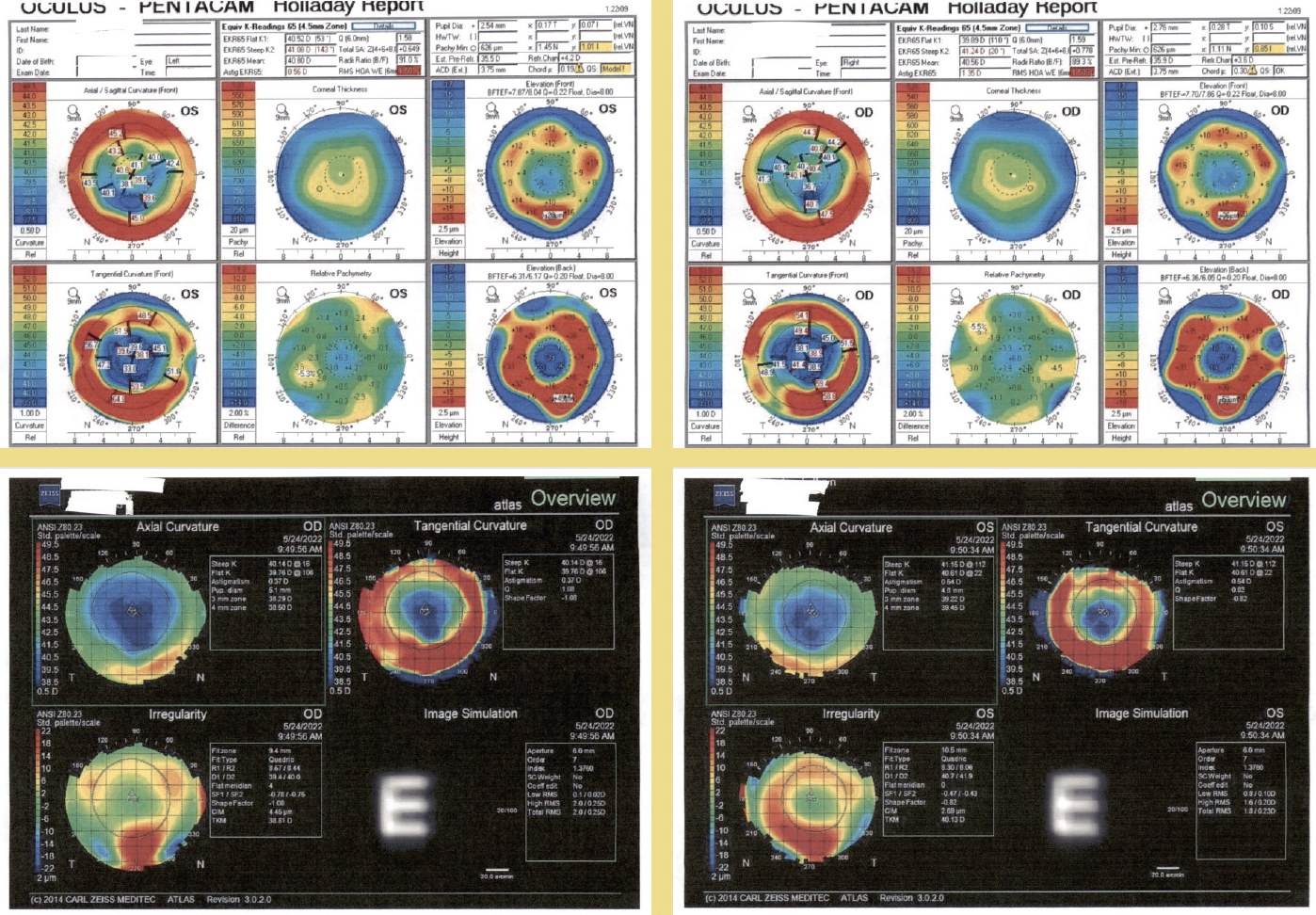
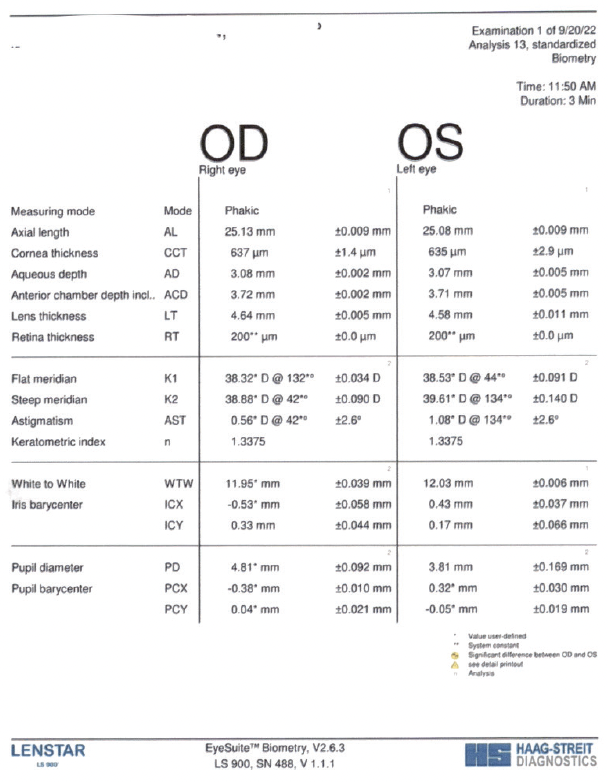
Figure. The patient’s preoperative testing.
After a thorough discussion, the patient elected to undergo cataract surgery and placement of an IC-8 Apthera (Bausch + Lomb) in the nondominant right eye. The procedure was uneventful, and initial results were satisfactory.
The possibility of some dimming of vision with bilateral implantation of an Apthera lens was discussed with the patient before surgery in her second eye. A simulation was conducted in the office with a pinhole lens in a trial frame. The patient elected to proceed with cataract surgery and implantation of an IC-8 Apthera owing to significant visual disturbances in the left eye. Surgery was uneventful.
At the most recent postoperative visit, the patient’s bilateral uncorrected distance and intermediate visual acuity was 20/20, and her bilateral uncorrected near visual acuity was 20/25. She reported no dimming of vision and was extremely satisfied with her result.
CRST: How often do you see patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, and what are some of the common complaints, complications, and challenges you face when treating these patients?
Dr. Fisher: A growing number of individuals who underwent PRK or LASIK at my or another practice 2 to 3 decades ago are presenting for care almost every day. They may report blurry vision, halos, glare, monocular diplopia, or a refractive shift. It is often important to perform additional testing, including corneal topography and tomography.
These tests can demonstrate anterior corneal surface irregularity consistent with irregular astigmatism causing visual blur, decentered ablations causing blurry vision or ghosting, and HOAs such as coma and trefoil.
CRST: How do you approach cataract surgery in patients with a history of RK, early PRK, or early LASIK?
Dr. Fisher: Patients with a history of refractive surgery who require cataract surgery can have high expectations and present unique challenges. It is important to counsel them on the increased difficulty of performing accurate IOL calculations and the possibility that further surgery, including refractive enhancements and IOL exchange, may be required. My preferred IOL power calculation formula for these patients is the Barrett True K.
To maximize spectacle independence, I have implanted multifocal IOLs in patients with a history of LASIK who have well-centered corneal ablations and little to no evidence of irregular astigmatism. I have also achieved successful results with multifocal IOLs in a small group of individuals whose RK procedures involved four incisions and a wide optical zone and whose topographic maps before cataract surgery showed little or no irregular astigmatism. My preference subsequently shifted to an EDOF IOL for both patient groups. More recently, however, I began to offer the IC-8 Apthera to many of these patients. I find small-aperture optics to be especially useful for individuals with high levels of corneal irregularity such as those with a history of RK and patients who experienced irregular healing after PRK or LASIK. I have no experience with the LAL, but this might also be an alternative for these patients.
I perform intraoperative aberrometry in most of my advanced technology IOL cases. Being able to refine the spherical power and control astigmatism is particularly important in patients who have a history of RK or LASIK. Interestingly, however, I do not find intraoperative aberrometry necessary if an IC-8 Apthera is to be implanted, owing to its mechanism of action, inherent tolerance of up to 1.50 D of astigmatism, and wide sweet spot in terms of lens power. Moreover, although I perform a manual or laser superficial keratectomy in patients with an irregular corneal surface who receive a multifocal IOL, I do not find the refractive procedure necessary if an IC-8 Apthera will be implanted.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK, early PRK, or early LASIK?
Dr. Fisher: This involves many of the same steps and technologies used for other individuals with glaucoma. Applanation tonometry, however, can produce artificially low readings in the former group because of postsurgical changes in the central cornea.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK, early PRK, or early LASIK?
Dr. Fisher: DED should be treated aggressively in this population. Any disruption of the normal tear film from insufficiency or instability is likely to have a greater impact on quality of vision than in someone with a virgin cornea. Treatment with artificial tears, punctal plugs, and topical medication to increase natural tear production can improve visual outcomes and enhance patient comfort.
1. Fu L, Patel BC. Radial keratotomy correction. StatPearls [Internet]. January 2022. PMID:32644588.
Common Complaints and Ideal Solutions
Careful evalution is key.
CRST: How often do patients with a history of RK surgery present to your practice, and what are some of the common complaints, complications, and challenges you face when treating these patients?
A. John Kanellopoulos, MD: RK was the drastic predecessor of a huge number of surgical procedures for myopic correction in the late 1980s and early '90s. Hundreds of thousands of patients in the United States and Europe were treated with RK, a technique developed in Russia by pioneering surgeon Slava Fyodorov, MD.
The most common complaint we have experienced as clinicians from this population is a long-term complication not anticipated by surgeons at the time. As the cornea ages and its biomechanical properties change, RK patients invariably experience a progressive myopic correction that exceeds the originally planned effect that renders them hyperopic. Most individuals who underwent RK present to ophthalmic practices now because they are experiencing visual function issues caused by the progressive hyperopia after enjoying reasonable visual function for sometimes decades. These patients often experience a diurnal variation with their vision that is worst in the morning, moderate in midday, and a little better at the end of the day.
Starbursts are another unwanted visual phenomenon after RK but one that was evident even during the first years after the procedure. Starbursts are attributed to light scatter at the most central part of the corneal vertex or the edges of the RK incisions. Once the pupil dilates at night, starbursts typically appear around a car’s headlights. Additional complaints these patients may have are DED, foreign body sensation, halos, and a poor quality of vision at night.
The main challenge in treating patients relates to the intrinsic biomechanical effect of the RK incisions. Because most incisions are incised through the limbus (classic Russian technique), it is impossible to halt diurnal variation in refraction without a limbus-to-limbus corneal transplant. The first step of management is therefore to study each patient’s diurnal variation carefully, discuss it with them, and ascertain at what time(s) of day they most desire crisp UCVA. The task can be hard because they generally do not understand that their situation differs from that of someone who underwent modern LASIK. For example, if the RK procedure was performed 30 years ago, the surgeon is dealing with at least three refractions over the course of a day instead of one. Most RK patients experience significant hyperopic shifts of 2.00 to 5.00 D. Some of them received arcuate incisions for astigmatism that, in the worst case scenario, intersected with the radial incisions or had more than eight radial incisions to treat higher amounts of myopia. Rarely, patients develop ectasia where the radial and arcuate incisions intersect and/or any of the incisions have perforated the cornea, resulting in higher corneal instability.
Once the refractive target is reached by studying the preferred daytime correction as noted earlier, the crystalline lens must be evaluated closely. Most patients who underwent RK are older than 50 years of age, so a fair number of them have or are developing cataracts. Lens-based correction, moreover, can be a reasonable treatment strategy even for those without cataracts. Advances in IOL calculations and lens technology have improved our ability to address refractive errors at the time of lens surgery. A caveat is that, if the effective central ablated area is slightly or grossly decentered, lens surgery cannot reverse the impact of that decentration on visual quality. The surgeon must determine whether laser vision correction could also offer significant improvement of the corneal optics in cases with a decentered ablation. We have reported the results of topography-guided laser ablation to recenter a decentered optical zone in previous RK.1
Some clinicians have evaluated whether CXL can reduce the diurnal refractive shifts RK patients experience during the day. A problem is that most CXL procedures address only the central 7 mm of the cornea. As mentioned earlier, the entire cornea (12 mm) is biomechanically unstable after RK. No currently available CXL device can treat to that diameter range.
In the rare instances when a post-RK patient develops ectasia, it is a result of weakness at the intersections of radial and arcuate incisions (as noted earlier), eye rubbing, or subtle keratoconus that was undetected before the original RK procedure (corneal topography did not even exist then). One option for treatment is to remove the epithelial plug and resuture the arcuate and radial incisions, wait 6 to 12 months for the corneal biomechanical stability to improve, and then perform either laser vision correction or a lens-based procedure. In extreme situations, corneal transplantation may be the only way to address corneal ectasia after RK. Generally, however, surface ablation and LASIK are safe and effective alternatives. If LASIK is chosen, it becomes challenging to enhance again in the future; lifting the flap again is not advised.
Phakic IOL implantation is an option, especially for patients with severe anisometropia, but again, diurnal variation is an issue. The advantage of a phakic IOL is that these individuals typically have deep anterior chambers because they were myopic before undergoing RK. In some cases, bioptics can be a successful strategy. In other words, laser treatment is performed to reshape and center the flattened area. Later, a phakic IOL is implanted to correct spherical error, astigmatism, and/or anisometropia.
None of the surgical strategies discussed in this section can eliminate starbursts and diurnal variation in refraction.
I have had experience with a large number of RK patients, both in the United States and Europe. In both settings, RK was the preferred treatment for myopic correction in the 1980s and '90s. The higher the myopia, the greater the challenge of managing the patient’s adverse effects. The surgeon must therefore decide whether the patient will benefit more from (1) a corneal topography-guided or ray-tracing procedure, possibly followed later by cataract surgery or (2) lens surgery to address both the cataract and ametropia.
Whichever strategy is pursued, the small and possibly decentered ablation will continue to affect the patient’s mesopic and scotopic visual function postoperatively. Moreover, despite advances in IOL formulas, refractive surprises are common in patients who underwent RK.
To guide my treatment decisions and improve results, I perform extensive diagnostic testing, including Placido disc topography, Scheimpflug tomography, epithelial mapping with anterior segment OCT, laser interferometry to document axial length and crystalline lens thickness, and anterior chamber depth.
CRST: How do you approach cataract surgery in patients who underwent RK or early LASIK?
Dr. Kanellopoulos: I favor the ASCRS online calculator. I also use the Holladay 1 formula because most of these patients are myopic. For the keratometry readings, I rely on the Pentacam (Oculus Optikgeräte) because it measures the total corneal power, both anterior and posterior. I also study the IOL power calculation as if the patient has a virgin cornea and add 2.00 D to the suggested IOL power. I find this technique correlates well with ASCRS data. The decision challenge comes when the ASCRS formula provides a large range of IOL power—sometimes more than 2.00 D!
It is difficult for these patients, in my opinion, to benefit from multifocal or EDOF IOLs because these lenses usually require pristine corneal optics.
CRST: If you use intraoperative aberrometry, what specific benefits does the technology offer for patients who underwent RK or early LASIK? Do you consider a two-step procedure with a laser ablation followed by cataract surgery for individuals who have irregular corneal astigmatism as a result of their previous refractive procedure?
Dr. Kanellopoulos: Intraoperative aberrometry can be advantageous for surgeons who are familiar with its use. A two-step procedure may be the optimal approach in patients with significant corneal irregularity and cataract. First, the irregularity is treated with excimer laser ablation. Later, cataract surgery is performed to address residual refractive error.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK or early LASIK?
Dr. Kanellopoulos: Assessment is difficult because the altered corneas compromise the accuracy of applanation tonometry. Furthermore, corneal optics can affect the accuracy of visual field testing and/or OCT assessment of peripapillary nerve fiber layer and ganglion cell mapping. The optic nerve evaluation is not just the most essential parameter to study in glaucoma; it may be the only reliable one in these cases.
A 70-year-old man presented to my office. The patient had undergone eight-incision radial keratotomy (RK) 40 years earlier and cataract surgery 2 years earlier (Figure 1), the latter of which resulted in a refractive surprise in his left eye. On presentation, his refraction was +1.50 -1.00 x 25º OS. His corrected distance visual acuity was 20/20, and his uncorrected distance visual acuity was 20/40. In addition to the ametropia, the patient reported severe visual problems, including glare and halos, in mesopic and scotopic conditions. Topography with the Pentacam (Oculus Optikgeräte) showed gross temporal and inferior decentration of the zone of corneal flattening from the RK (Figures 2 and 3).
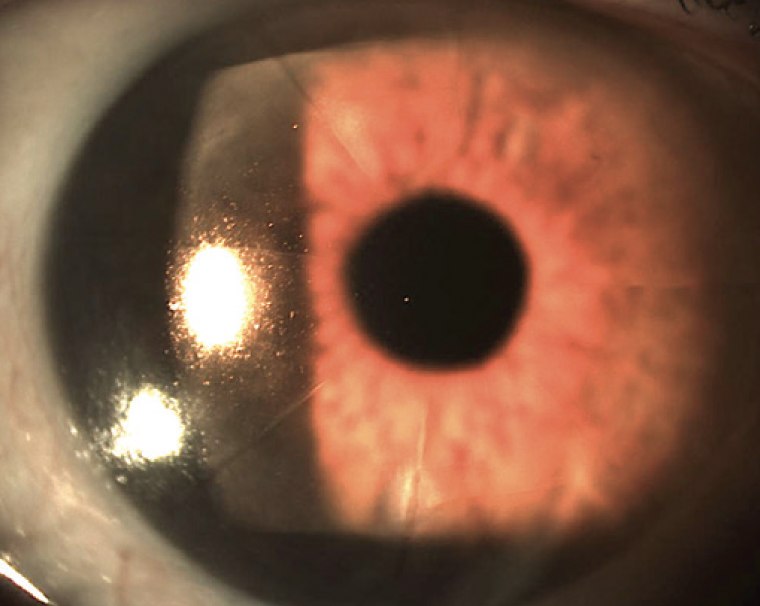
Figure 1. Slit-lamp image of the cornea andanterior segment before LASIK surgery. The eight-incision RK extending through the limbus (Russiantechnique) is clearly visualized.
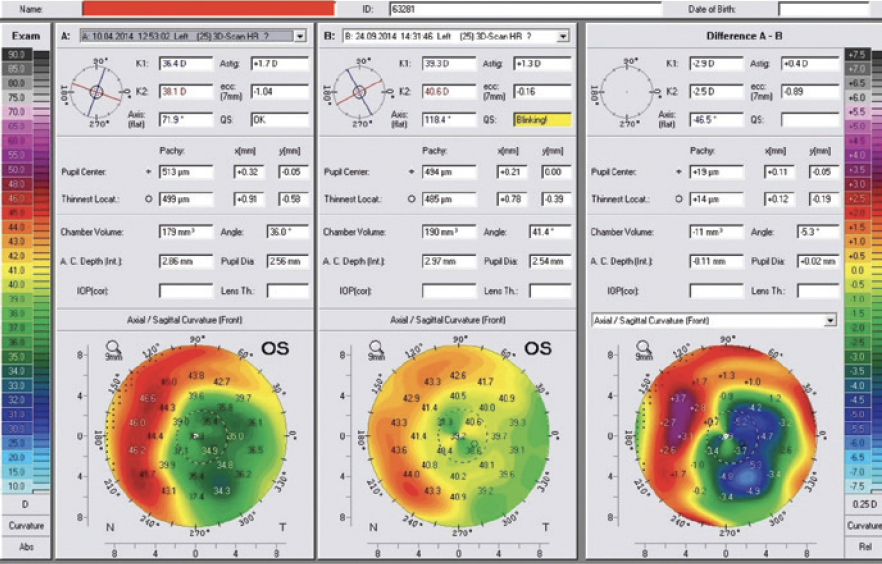
Figure 2. Preoperative imaging with Placido disctopographydocumented severe RK flattening anddecentrationtemporally.
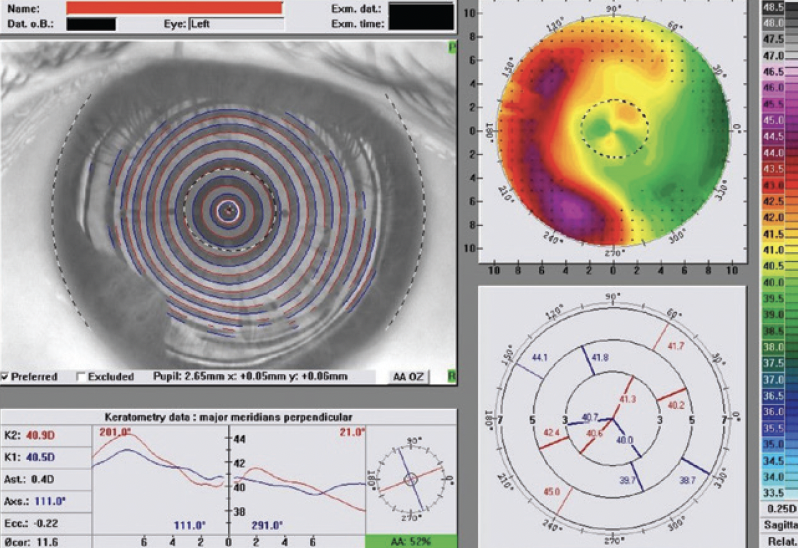
Figure 3. Preoperative imaging with Scheimpflug tomographymirrors the findings of Figure 2.
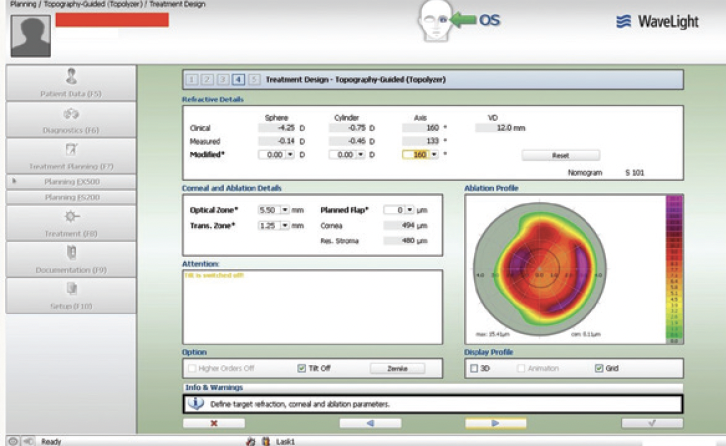
Figure 4. The planning treatment design first with refractive error set to 0 underlines the topographically guided attempt to normalize the decentration effect.
Topography-guided femtosecond LASIK with the WaveLight FS200 and EX500 excimer laser (both from Alcon) was performed to address the patient’s refractive error. The 0 refraction treatment plan illustrates the software’s zone recentering attempt (Figures 4 and 5). The astigmatic power and axis entered in the treatment plan matched those measured with topography and were in accordance with the topography-modified refraction my colleagues and I have introduced and reported.1,2 The postoperative treatment report and results are shown in Figures 6 through 8.
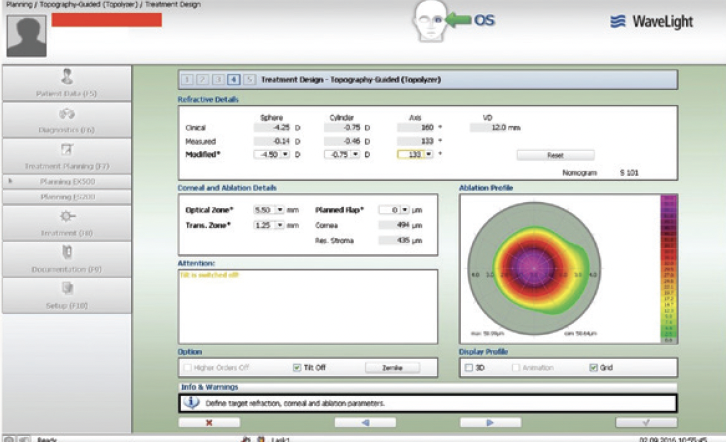
Figure 5. The planning treatment design with the refractive error.
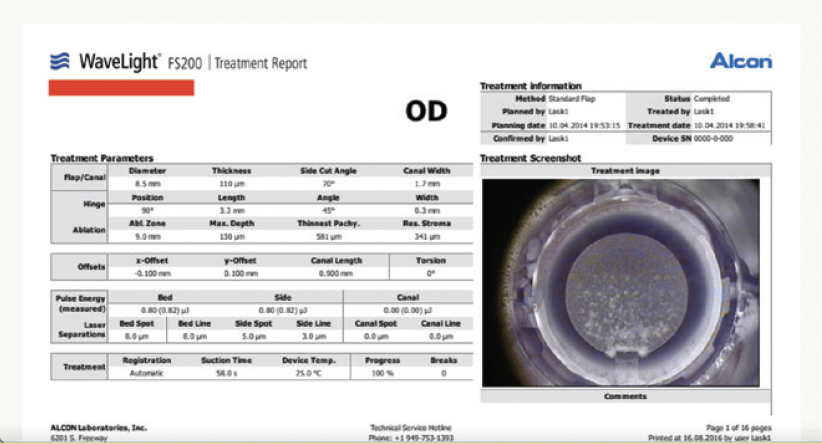
Figure 6. The postoperative treatment report from the WaveLight FS200 documents successful completion through the part of the cornea that has the radial incisions.
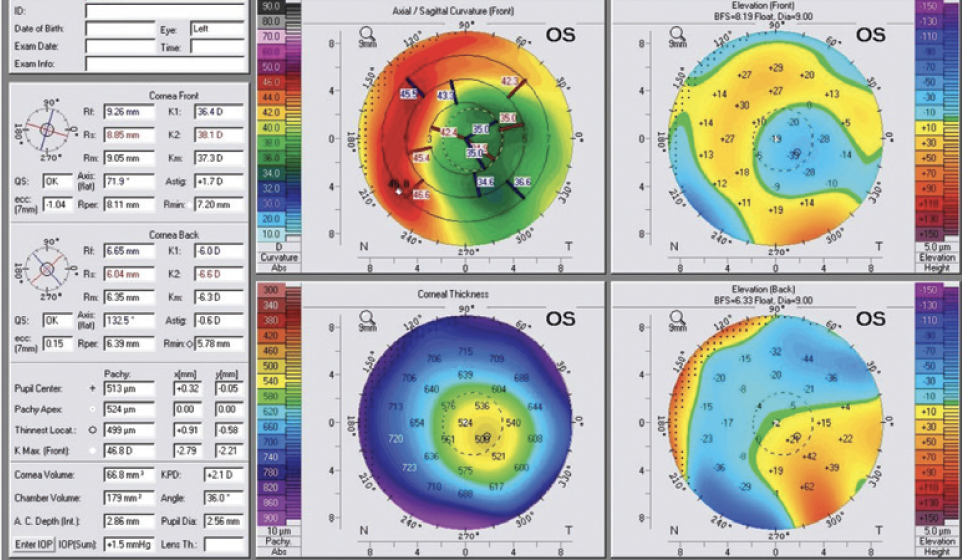
Figure 7. Pre- and post-LASIK Pentacam comparison and difference maps. The preoperative image mirrors the Placido topography decentration (A), the postoperative image confirms the recentration of the effective flattened area (B), and the difference of the preoperative minus the postoperative measurements confirms that the excimer plan noted above was effective and accurate (C).
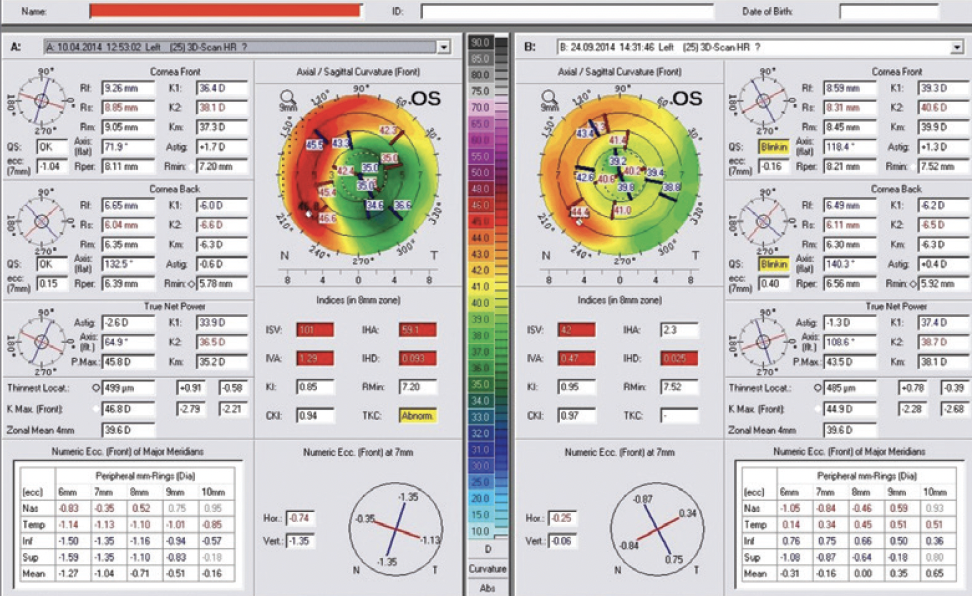
Figure 8. The topometric indices derived from pre- and postoperative Pentacam imaging documenting improvement, most importantly with the index of height decentration.
1. Kanellopoulos AJ. Topography-guided custom retreatments in 27 symptomatic eyes. J Refract Surg. 2005;21(5):S513-S518.
2. Kanellopoulos AJ. Topography-modified refraction (TMR): adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol. 2016;10:2213-2221.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK or early LASIK?
Dr. Kanellopoulos: My approach is the same as for any eye with OSD. It includes topical cyclosporine A, warm compresses, and tetracycline-type medications for blepharitis. I feel fortunate to have access to a newly available medication, doxycycline 40 mg (Oracea, Galderma). In my experience, its unique absorption properties in the gastrointestinal tract eliminate many of the adverse reactions patients can experience with doxycycline or minocycline use. My patients who administer the drug daily have had minimal to no adverse reactions, and I have found it effective for treating rosacea and blepharitis, which are the major causes of OSD symptoms in my practice.
1. Kanellopoulos AJ. Topography-guided custom retreatments in 27 symptomatic eyes. J Refract Surg. 2005;21(5):S513-S518.
Individuals With a History of RK or Early LASIK Can Be Easygoing Patients
Over the years, they have become accustomed to their suboptimal vision.
CRST: How often do patients with a history of RK surgery present to your practice, and what are some of their common complaints?

Hercules Logothetis, MD: I see maybe one or two a month. Common complaints include blurred and fluctuating vision, DED, and nighttime optical aberrations.
CRST: What challenges does treating these patients present?
Dr. Logothetis: Maximizing their quality of vision and tracking fluctuations in their refractions or glasses prescriptions can be challenging. Individuals with a history of RK, however, tend to be some of my most easygoing patients because they have become somewhat accustomed to not having the highest quality of vision, whereas some other patients want everything to be 10 out of 10.
Case Example
A 56-year-old woman underwent an eight-cut radial keratotomy performed in both eyes 25 years ago. Upon presentation, she reported that her vision was becoming progressively blurry in her left eye. In the same eye, she had a childhood history of mild amblyopia and a recent history of vitreous hemorrhage that required a vitrectomy about 1 year ago. Since that time, the patient had developed a cataract. Preoperative tomography is found in Figure 1. The manifest refraction in her right and left eyes was plano (20/20) and -6.00 +0.75 x 80º (20/40), respectively. The lens was clear in her right eye, but in her left eye, there was a grade 2 to 3 nuclear sclerotic cataract.
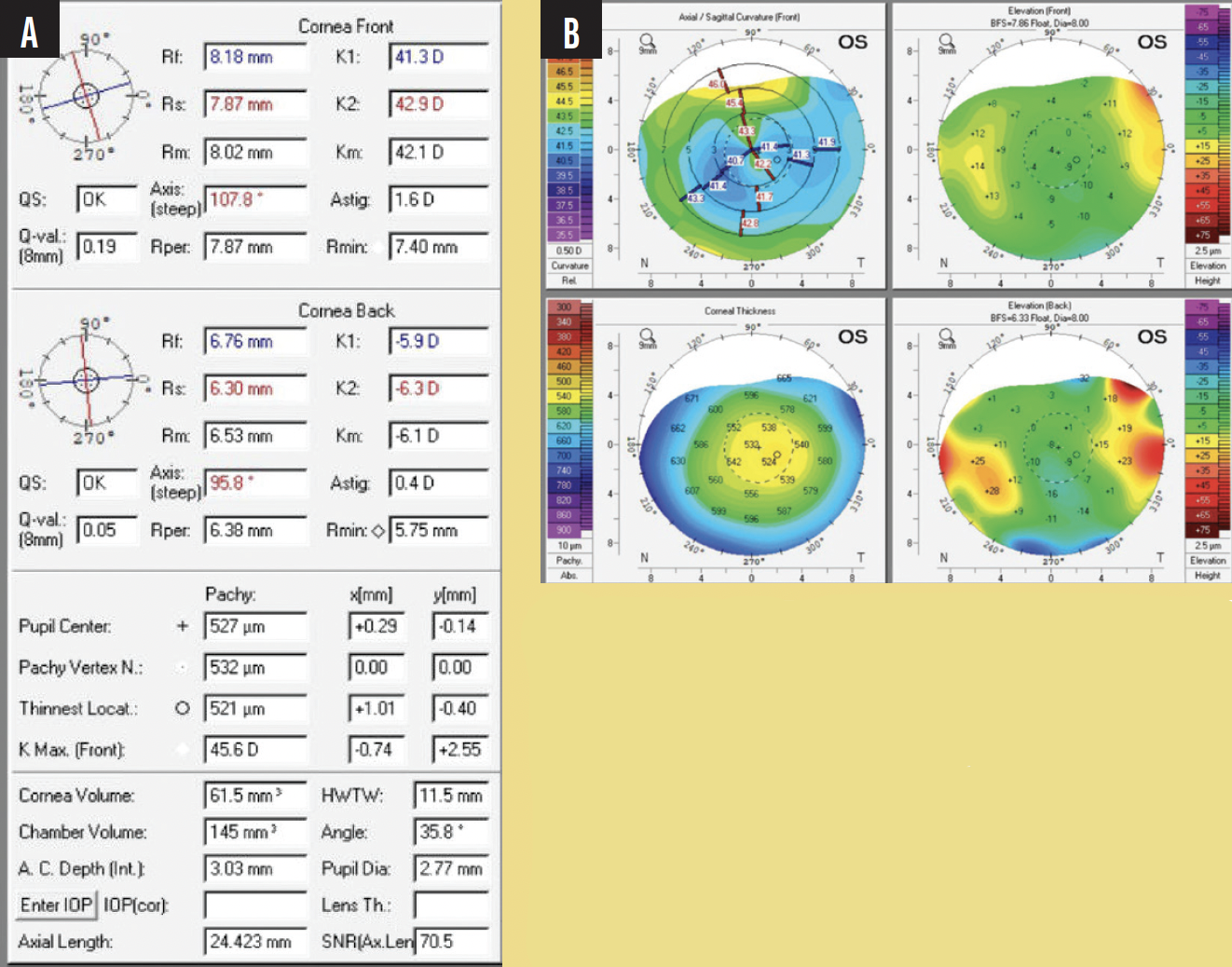
Figure 1. Preoperative tomography (A and B).
After discussing options with the patient, we decided on a monofocal IOL. A 21.00 D Tecnis Monofocal (ZCB00, Johnson & Johnson Vision) was implanted with a target of -0.50 D. I used the ASCRS calculator to select the IOL power (Figure 2). At postoperative month 1, her BCVA was 20/30. The patient was happy with her outcome.
Figure 2. IOL power calculations.
CRST: How do you manage patients who experience a progressive hyperopic shift after RK?
Dr. Logothetis: I observe these patients over time. They are refracted and evaluated with tomography every 6 months or so. Their glasses and/or contact lens prescriptions are updated as needed, and cataract development is monitored.
CRST: How do you manage patients who have developed ectasia after RK?
Dr. Logothetis: It depends largely on their age and BCVA. If patients experience obvious ectatic progression during observation, I typically refer them out for CXL. Most of them are old enough, however, that their corneas have essentially undergone natural CXL, so my goal is to improve their BCVA—be it with glasses, contact lenses, or cataract surgery.
CRST: How do you manage patients who have corneal scars or Salzmann nodular degeneration that requires surgery after RK?
Dr. Logothetis: I am not a corneal specialist. If a patient requires surgery for Salzmann nodules, a referral is made to another provider.
CRST: Would you consider a phakic IOL for an RK patient with continuous hyperopia? If so, please describe your approach and/or share an example.
Dr. Logothetis: I implanted the Visian ICL as a fellow but do not currently implant the lens. Because most RK patients are older, I assume that they are not candidates for the technology for that reason. Most refractive and cataract surgeons I know stop implanting phakic IOLs after a patient reaches 45 years of age. The short answer is I would not implant a phakic IOL in this patient population.
CRST: Would you consider RLE in a patient with a history of RK?
Dr. Logothetis: Yes, I would, just as I would consider cataract surgery if they had cataracts.
CRST: How often do you see patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, and what are some of the common complaints, complications, and challenges you face when treating these patients?
Dr. Logothetis: Early adopters of LASIK tend to be a little more easygoing and have more reasonable expectations than modern LASIK patients. The former do not complain all that much and, for the most part, are strong supporters of the procedure. The unwanted phenomena they report include nighttime glare and halos (more significant than with modern LASIK) and persistent DED. It is also more common to see epithelial ingrowth in this population because of how LASIK flaps were made at that time. Flap melts, however, and corneal irregularity as a result of epithelial ingrowth are rare.
CRST: Is an additional workup required in these patients, for example, to detect small, irregular, or decentered ablations; HOAs; or epithelial basement membrane dystrophy?
Dr. Logothetis: I approach these patients much as I do other patients. A full roster of diagnostic testing is performed, including analysis with the Pentacam, biometry measurements with the IOLMaster (Carl Zeiss Meditec), an assessment with the HD Analyzer (Visiometrics), OCT imaging, refraction, and autorefraction. I also carefully evaluate the eyes for decentered ablations and HOAs.
CRST: How do you approach cataract surgery in patients with a history of RK or early LASIK?
Dr. Logothetis: Again, I approach these patients much as I do other patients. They receive a complete examination. If necessary, a hard contact lens overrefraction is performed.
I spend a lot of time explaining to these patients the challenges that their history of refractive surgery brings to cataract surgery so that their expectations are realistic. I emphasize that they will depend on glasses to some extent postoperatively, even if only for reading or driving at night.
I also assume that these patients’ quality of vision has been reduced somewhat by their corneal procedure.
CRST: Would you implant a multifocal IOL in a patient who has a history of RK or early LASIK?
Dr. Logothetis: I would not. I think it would add too many variables to an eye that is already complex.
CRST: Would you implant a small-aperture IOL in a patient who has a history of RK or early LASIK?
Dr. Logothetis: I gained some experience with small-aperture IOLs as a fellow, but I do not actively use the technology. I would, however, be willing to implant one in the patient’s nondominant eye.
CRST: Would you implant an LAL in a patient who has a history of RK or early LASIK?
Dr. Logothetis: As a fellow, I worked with the LAL as part of the clinical trials. I think it can be an excellent option for these patients if they are willing to put in the time required for light adjustments and wear the protective eyewear. The LAL is probably the best choice for this population from my perspective.
CRST: What is your preferred IOL power calculation for patients who underwent RK or early LASIK?
Dr. Logothetis: I tend to use the ASCRS postrefractive online calculator.
CRST: If you use intraoperative aberrometry, what specific benefits does the technology offer for patients who underwent RK or early LASIK?
Dr. Logothetis: I lean toward the use of the ORA System (Alcon) for these patients. The additional measurements increase my confidence. Preoperative biometry measurements are performed with the Pentacam and IOLMaster, and the results obtained with the ORA are used as a tiebreaker if the first two devices disagree on the IOL to implant.
CRST: Do you consider a two-step procedure with a laser ablation followed by cataract surgery for individuals who have irregular corneal astigmatism as a result of their previous refractive procedure?
Dr. Logothetis: I suppose I would consider referring a patient out for topography-guided ablation if I felt the procedure were required to achieve the best result, but I have not had to address the issue yet.
CRST: How would you perform a glaucoma assessment of patients who underwent RK or early LASIK?
Dr. Logothetis: My approach would be the same as for any patient with glaucoma. The disease is multifaceted, so several variables must be tracked over time. Pachymetry, tonometry, gonioscopy, visual field testing, and OCT imaging would be performed. If the patient has a history of refractive surgery, I would bear in mind that IOP measurements might be inaccurate, but I think glaucoma can be monitored well with visual field testing, OCT analysis, and an examination of the optic nerve’s appearance.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK or early LASIK?
Dr. Logothetis: My tips don't differ from well-established guidelines for the general DED population. My patients undergo matrix metalloproteinase-9 testing, tear osmolarity, an assessment with the HD Analyzer, an evaluation of tear breakup time, and fluorescein staining.
In addition to over-the-counter medications, I prescribe cyclosporine ophthalmic emulsion 0.09% (Cequa, Sun Ophthalmics), lifitegrast ophthalmic solution 5% (Xiidra, Novartis International), and cyclosporine ophthalmic emulsion 0.05% (Restasis, Allergan) and offer treatment with devices such as the LipiFlow Thermal Pulsation System (Johnson & Johnson Vision).
Recommended Treatments Depend on Patient Demographics and Expectations
Setting appropriate goals is key.
CRST: What are some of the common complaints, complications, and challenges you face when treating patients with a history of RK surgery?


Eike Matthiessen, MD, and Lena Beckers, MD: RK delivered effective results initially but demonstrated limited predictability, especially in the long term. Many patients presented with over- or undercorrections years after surgery. Individuals with high myopia before their surgery have tended to develop a hyperopic shift approximately 10 years after RK in addition to diurnal fluctuations in vision, halos, and glare.
CRST: How do you manage patients who experience a progressive hyperopic shift after RK?
Drs. Matthiessen and Beckers: It depends on their age and expectations. Spectacles can be prescribed. If patients cannot tolerate spectacles, are older than 45 years of age, and have presbyopia, then an RLE can be considered.
Various diagnostic technologies provide valuable information about the cornea to guide IOL selection. It is important to explain to patients that no IOL can eliminate diurnal fluctuations in vision. Reversing a hyperopic shift and restoring emmetropia, however, can improve their quality of life, as can reducing halo and glare symptoms that are caused by the RK, a cataract, or tear film instability.
If the patient is young and corneal thickness is adequate, PRK is a safe and effective option for managing secondary ametropia after RK. If the refraction is stable, the crystalline lens is clear, and the anterior chamber is deep, a phakic IOL is an alternative.
CRST: How do you manage patients who have developed ectasia after RK?
Drs. Matthiessen and Beckers: Management depends on the stage of keratectasia, patient expectations, and goal setting. If the keratectasia is in the early stages and the patient retains usable vision, we consider performing CXL according to the Athens protocol in combination with topography-guided PRK. For more advanced disease, if management with contact lenses is inadequate and CXL seems unpromising, surgical intervention is discussed.
CRST: How do you manage patients who have corneal scars or Salzmann nodular degeneration that requires surgery after RK?
Drs. Matthiessen and Beckers: We have not yet encountered a patient with Salzmann nodular degeneration after RK.
CRST: Would you consider a phakic IOL for an RK patient with continuous hyperopia? If so, please describe your approach and/or share an example.
Drs. Matthiessen and Beckers: Phakic lens implantation is an effective approach to treating refractive errors after RK because the procedure affects the biomechanical stability of the cornea less than corneal refractive surgery does. Refractive stability should be established before surgical intervention.
Most patients were myopic before undergoing RK, so a deep anterior chamber is to be expected. The cornea, however, flattens after RK. If a hyperopic shift has occurred, it is important to measure anterior chamber depth to ensure it is adequate for phakic IOL implantation.
CRST: Would you consider RLE in a patient with a history of RK?
Drs. Matthiessen and Beckers: Definitely. We prefer the Liberty2 (1stQ) because it is easy to explant the add-on lens if a patient does not tolerate the diffractive optic. We ensure that preexisting HOAs are not a contraindication for a diffractive multifocal optic. If the amount is greater than 0.35 µm, we choose a monofocal IOL. We discuss the pros and cons of all IOL options in detail with patients. We are also careful to determine if they are experiencing diurnal refractive changes or bothersome halos and glare that cannot be attributed to a cataract, in which case a monofocal IOL is often the best choice.
Four points to bear in mind when approaching RK patients are as follows:
No. 1. They typically underwent RK 20 years ago.
No. 2. They likely have presbyopia.
No. 3. They might have experienced a hyperopic shift.
No. 4. They are generally wearing glasses—something they sought to avoid when they underwent RK.
CRST: How often do you see patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, and what are some of the common complaints, complications, and challenges you face when treating these patients?
Drs. Matthiessen and Beckers: Even in the 1990s or 2000s, LASIK was safe, effective, and predictable, although the technology is more advanced today.
Once in their 40s, patients usually complain about wearing reading glasses. They often request RLE and multifocal IOLs. Their desire for spectacle independence makes them challenging to satisfy. A careful assessment is required to detect HOAs and corneal irregularities.
We prefer the Liberty2 system in post-LASIK eyes; the multifocal add-on IOL can be explanted easily if the patient cannot tolerate multifocality or is dissatisfied with the result. It is important to discuss the possibility of an explantation during preoperative counseling.
CRST: Is an additional workup required in these patients, for example, to detect small, irregular, or decentered ablations; HOAs; or epithelial basement membrane dystrophy?
Drs. Matthiessen and Beckers: In our practice, every patient receives a detailed preoperative examination. That said, it is especially crucial for patients with a history of LASIK who might be more critical of their results than other patients. When a patient expresses dissatisfaction with their results, we discuss them with the patient and do not recommend a multifocal/add-on IOL.
CRST: Would you implant a multifocal IOL in a patient who has a history of RK or early LASIK?
Drs. Matthiessen and Beckers: Most individuals who underwent RK have HOAs or corneal irregularities, making a multifocal IOL a suboptimal choice. Progressive flattening of the central cornea often causes a hyperopic shift and renders the long-term visual outcome of cataract surgery unpredictable. Moreover, refractive instability, diurnal fluctuations in vision, and halos and glare are common in this population. That said, with careful patient selection, some of these individuals can benefit from a multifocal IOL.
It is essential to determine if halo and glare symptoms cannot be attributed to cataract and diurnal refractive changes. Less than 2 years of refractive stability is a contraindication for a multifocal IOL because there is a high risk that refractive changes will occur after surgery. Achieving an emmetropic outcome is important in patients who desire spectacle independence with a multifocal IOL.
Among the measurements we assess are total refraction, wavefront refraction, and topography. We repeat these measurements 6 to 12 months apart. A review of these data helps us decide on the intervention that will benefit the patient most.
CRST: Would you implant a small-aperture IOL in a patient who has a history of RK or early LASIK?
Drs. Matthiessen and Beckers: The decision depends on the preoperative assessment. The lens design can minimize halos and glare, for example, in someone with a history of LASIK. We have not, however, used the approach yet.
CRST: Would you implant an LAL in a patient who has a history of RK or early LASIK?
Drs. Matthiessen and Beckers: We want to see greater predictability reported before we use the technology for these patients.
CRST: What is your preferred IOL power calculation for patients who underwent RK or early LASIK?
Drs. Matthiessen and Beckers: We favor the Haigis formula and have developed our own nomogram.
CRST: If you use intraoperative aberrometry, what specific benefits does the technology offer for patients who underwent RK or early LASIK? Do you consider a two-step procedure with a laser ablation followed by cataract surgery for individuals who have irregular corneal astigmatism as a result of their previous refractive procedure?
Drs. Matthiessen and Beckers: We do not use intraoperative aberrometry in our clinic. Topography-guided PRK can achieve good results in eyes with irregular corneal astigmatism if the refraction is stable and corneal thickness is adequate.1 The risks and benefits of any surgery must be considered carefully before proceeding.
Case Example
A 60-year-old man presented with a complaint of a bilateral decrease in vision. He had undergone radial keratotomy on the left eye in the late 1980s and cataract surgery with an iris-fixated anterior chamber IOL in the right eye. He said his vision, particularly in the left eye, had been worsening for years.
An examination revealed a spherical equivalent and an axial length of -8.25 D and 27.27 mm OD and -5.75 D and 26.48 mm OS. The patient’s uncorrected distance visual acuity (UDVA) was 0.0 logMAR (decimal 1.0) OD and 0.7 logMAR (decimal 0.2) OS. His best corrected distance visual acuity was 0.0 logMAR (decimal 1.0) with a refraction of 0.00 -1.50 x 1º OD and 0.0 logMAR (decimal 1.0) with a refraction of +3.50 -1.50 x 110º OS. A slight nuclear cataract was evident in each eye at the slit lamp.
The patient’s situation was typical of someone with a history of radial keratotomy. He had a hyperopic shift, poor UDVA, diurnal refractive changes, a difference in IOP elevation between eyes (15 mm Hg OD vs 20 mm Hg OS), and a difference in anterior chamber depth. Average keratometry readings were 44.03 D OD and 32.37 D OS. Corneal thickness was slightly greater than normal on the vertex (576 µm OD and 586 µm OS). The patient also had high expectations; he desired spectacle independence and requested a multifocal IOL based on research he had done.
His subjective refraction was measured twice with a separation of 8 weeks between measurements. Topography was also repeated. Based on the preoperative findings, a monofocal toric IOL (1stQ) was implanted. After surgery, the IOP was 14 mm Hg OU. The anterior chamber depth was 2.82 mm OD and 3.84 mm OS. The patient’s UDVA was 0.3 logMAR (decimal 0.5), and the refraction of the eye was 0.00 -1.25 x 106º. Diurnal refractive changes persisted. The patient was satisfied with his UDVA but dissatisfied with his uncorrected near visual acuity.
Three months after surgery, the right eye had 0.0 µm spherical aberrations and 0.05 µm higher-order aberrations, and the left eye had 0.09 µm spherical aberrations and 0.16 µm higher-order aberrations. The pros and cons of a multifocal IOL were discussed at length. An IOL exchange for a Liberty2 (1stQ) with a 2.25 D add-on lens was performed in the left eye. At 1 day and 1 week after surgery, the patient’s best corrected distance visual acuity was 0.3 logMAR (decimal 0.5) OS, and he was able to read without glasses. Two months after surgery, his UDVA was 0.2 logMAR (decimal 0.6) OS, and his binocular UDVA was 0.0 logMAR (decimal 1.0). His uncorrected intermediate visual acuity was 0.4 logMAR (decimal 0.4) OS, and his binocular uncorrected intermediate visual acuity was 0.2 logMAR (decimal 0.63). His uncorrected near visual acuity was 0.4 logMAR (decimal 0.4) OS, and his binocular uncorrected near visual acuity was 0.3 logMAR (decimal 0.5).
The patient was happy with the results. Neural adaptation to the multifocal add was quicker than we had expected. He noticed but was not surprised or worried about a reduction in contrast sensitivity.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK or early LASIK?
Drs. Matthiessen and Beckers: Pachymetry is useful, particularly after RK, because the irregular shape of the cornea can render applanation tonometry inaccurate. We also perform gonioscopy; OCT with analyses of the retinal nerve fiber layer and ganglion cell density, especially in the macula; visual field testing; and funduscopy in mydriasis with close evaluation of the cup and rim. We advise patients to return for a glaucoma assessment at least yearly if no signs of disease or progression are found.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK or early LASIK?
Drs. Matthiessen and Beckers: We start conservatively by recommending the instillation of preservative-free artificial tears at least three times a day, the nightly application of an ointment, and the use of warm compresses and soft massage of the eyelid margin twice a day. We also recommend smoking cessation and the treatment of inflammatory diseases such as rosacea and blepharitis. If these measures prove inadequate, additional options are pursued, including treatment with the LipiFlow Thermal Pulsation System and intense pulsed light therapy.
1. Filev FS, Kromer R, Frings A, Dragneva D, Mitov T, Mitova D. Photorefraktive keratektomie (prk) zur korrektur der restfehlsichtigkeit nach radiärer keratotomie. Klin Monbl Augenheilkd. 2020;237(8):961-967.
A Scleral Lens Can Be Advantageous in Complex Cases
Comprehensive cataract surgeons should consider this option in appropriate cases.
CRST: How do you manage patients who experience a progressive hyperopic shift after RK?

Marguerite B. McDonald, MD, FACS: If their pre-RK refraction is known and they have -4.00 D of myopia or less with a healthy retina on a dilated fundus examination, pilocarpine HCl ophthalmic solution 1.25% (Vuity, Allergan/AbbVie) is an option. A custom contact lens with a small aperture is also an option if they can tolerate it. Many of these patients are old enough to have cataracts, so phacoemulsification with implantation of an IC-8 Apthera is another option.
CRST: How do you manage patients who have developed ectasia after RK?
Dr. McDonald: It is typical for the cornea to stabilize eventually and for ectatic progression to halt. There are data supporting the use of CXL in this situation.
CRST: How do you manage patients who have corneal scars or Salzmann nodular degeneration that requires surgery after RK?
Dr. McDonald: Clinically significant Salzmann nodules should be removed. Three months later, if the corneal shape has stabilized, biometry measurements are obtained, and cataract surgery is scheduled.
CRST: Would you consider a phakic IOL for an RK patient with continuous hyperopia? If so, please describe your approach and/or share an example.
Dr. McDonald: Virtually all patients who underwent RK are old enough to have clinically significant cataracts. I would prefer to perform cataract surgery and implant an IC-8 Apthera in this situation.
CRST: Would you consider RLE in a patient with a history of RK?
Dr. McDonald: Again, these patients are old enough to have clinically significant cataracts that should meet insurers’ criteria for removal.
CRST: In patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, is an additional workup required, for example, to detect small, irregular, or decentered ablations; HOAs; or epithelial basement membrane dystrophy?
Dr. McDonald: I obtain virtually all the information I need from the Pentacam; on rare occasions, I also perform a hard contact lens overrefraction.
CRST: Would you implant a multifocal IOL in a patient who has a history of RK or early LASIK?
Dr. McDonald: I usually do not recommend this technology because the patient’s corneal optics are already compromised. Moreover, based on their age, these individuals often have significant OSD.
CRST: Would you implant a small-aperture IOL in a patient who has a history of RK or early LASIK?
Dr. McDonald: Yes, this lens is appropriate for both patient groups.
CRST: Would you implant an LAL in a patient who has a history of RK or early LASIK?
Dr. McDonald: I might consider an LAL for a patient who underwent an early iteration of LASIK. I would choose an IC-8 Apthera for a patient who underwent RK because these individuals often experience diurnal variation in their refraction that cannot be addressed by an LAL.
Case Examples
By Gerard D'Aversa, OD, and Marguerite B. McDonald, MD, FACS

Comprehensive cataract surgeons should consider a scleral lens as an option for their complex cases. Some patients would benefit from this intervention postoperatively such as those who continue to have suboptimal optics and good manual dexterity. These patients must be told preoperatively that this is a possibility. The following cases are perfect examples.
Case No. 1
A 37-year-old man with post-LASIK ectasia presented with the chief complaint of worsening vision. Figure 1 shows prefitting imaging of the eyes. His BCVA was 20/40 OD and 20/60 OS, his manifest refraction was +2.25 -1.00 x 145º OD and +4.25 -1.75 x 80º OS, and the eyes were stable for more than 2 years. After Pentacam (Oculus Optikgeräte) images were obtained and the patient was counseled about his options, we decided to proceed with scleral lenses in both eyes. A Zenlens oblate scleral lens (Bausch + Lomb) with a base curve of 9 mm, a diameter of 16 mm, and a sagittal depth of 4.7 mm was implanted in the right eye, and a Zenlens oblate toric scleral lens with a base curve of 9.5 mm, a diameter of 16 mm, and a sagittal depth of 4.5 mm was implanted in the left eye.
Figure 1. Prefitting images of the right (A, B) and left (C, D) eyes of the patient in Case No. 1.
At his first scleral lens examination about 1 month later, the patient had a significant improvement in visual acuity (20/20 OD and 20/25 OS). The lenses could be successfully inserted and removed with standard scleral plungers.
Case No. 2
A 58-year-old man with a history of radial keratotomy presented to our office with a chief complaint of significant blurry vision. He had tried hybrid contact lenses in the past without success.
On examination, the patient had irregular astigmatism from the radial keratotomy in both eyes, ocular hypertension in his right eye, and a horseshoe tear in his left. His BCVA was 20/60 OD and 20/60 OS. Prefitting imaging is shown in Figure 2.
Figure 2. Prefitting images of the right (A, B) and left (C, D) eyes of the patient in Case No. 2.
We decided to give scleral lenses a try to improve his vision. Pentacam images were obtained. A Zenlens oblate scleral lens with a diameter of 16 mm and sagittal depth of 4.7 mm was implanted in the right eye, and a Zenlens oblate scleral lens with a base curve of 8 mm and a diameter of 16 mm was implanted in the left eye. After finalizing his scleral lenses, his BCVA improved to 20/25 OU.
Case No. 3
A 42-year-old man with a history of retinitis pigmentosa in both eyes and stable post-LASIK ectasia in the right eye presented for a scleral evaluation. The patient was not comfortable with any glasses prescription. He wore soft contact lenses in both eyes, but they did not provide good vision in his right eye. On examination, his BCVA was 20/80 OD, and the manifest refraction was +1.50 -4.00 x 35º OD. Figure 3 shows the prefitting imaging with the Pentacam.
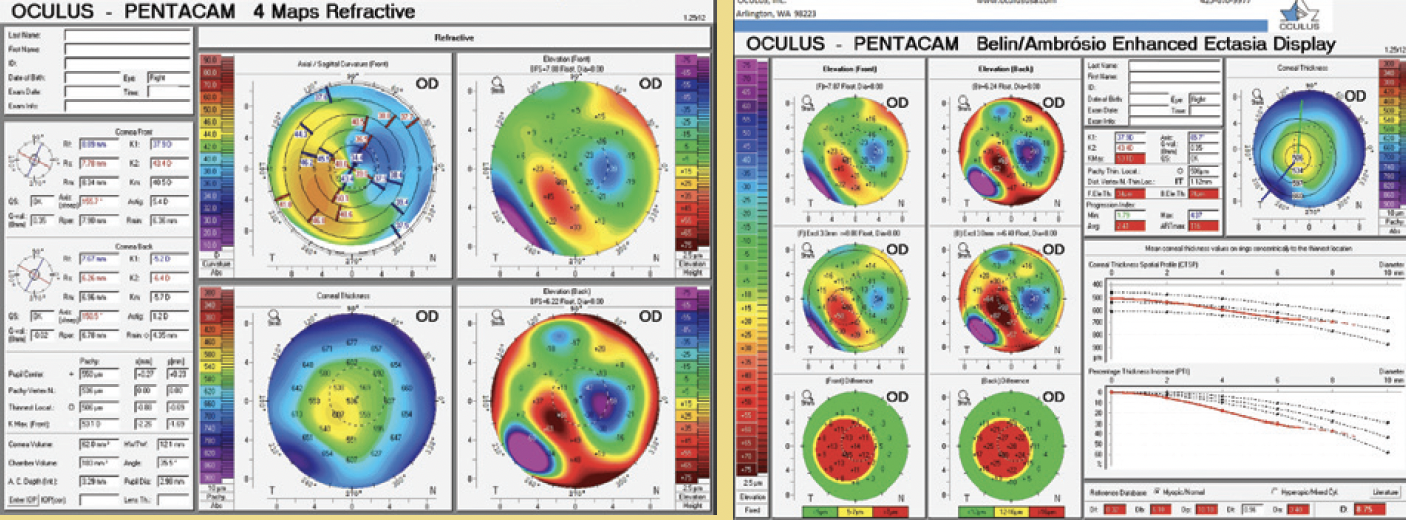
Figure 3. Prefitting images of the right eye of the patient in Case No. 3.
The patient was fit for a scleral lens in his right eye. An oblate Zenlens with a base curve of 9 mm, a diameter of 16 mm, and a sagittal depth of 4.75 mm was selected. With the scleral lens, his BCVA was limited by the retinitis pigmentosa but still improved to 20/50, and the patient reported a significant subjective improvement in acuity. He was able to insert the scleral lens successfully with the aid of the SeeGreen device (Dalsey Adaptives).
CRST: What is your preferred IOL power calculation for patients who underwent RK or early LASIK?
Dr. McDonald: The Barrett postrefractive surgery formulas on the IOLMaster and ASCRS website are my favorites, but I use intraoperative aberrometry as well.
CRST: What specific benefits does intraoperative aberrometry offer for patients who underwent RK or early LASIK?
Dr. McDonald: I typically observe agreement within ±0.50 D between my measurements. If discordance occurs, I choose the strategy that yields the most myopic result.
CRST: Do you consider a two-step procedure with a laser ablation followed by cataract surgery for individuals who have irregular
corneal astigmatism as a result of their previous refractive procedure?
Dr. McDonald: I generally prefer to perform cataract surgery first and address any irregular astigmatism later. Implanting an IC-8 Apthera should minimize or eliminate irregular corneal astigmatism.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK or early LASIK?
Dr. McDonald: I basically use my standard approach. Because myopic LASIK reduces corneal thickness, however, I use a corneal thickness IOP correction chart to improve the accuracy of applanation tonometry in this subset of eyes.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK or early LASIK?
Dr. McDonald: As noted earlier, the optical system of these eyes has already been compromised by their refractive surgery. OSD compounds the problem. Treatment must be aggressive and sustained. Patients, moreover, must be counseled that DED can be controlled but not cured.
Treating the Ocular Surface and Other Tips for Successful Outcomes
Aim to remove variables from a complex situation.
CRST: How often do patients with a history of RK surgery present to your practice, and what are some of the common complaints, complications, and challenges you face when treating these patients?


Neel S. Vaidya, MD, MPH, and Parag A. Majmudar, MD: Because of the popularity of RK in the 1980s and '90s, a growing number of patients are now seeking care for a variety of visual complaints—from progressive hyperopia to presbyopia to cataracts. They desire refractive cataract surgery and a restoration of spectacle independence. Helping them can be challenging, and it often requires a multifaceted approach. In addition to the typical demands of refractive surgery patients, RK patients have experienced optical changes induced by the radial incisions. Instability of those incisions, moreover, can create diurnal fluctuations and make it difficult to hit the refractive target.
CRST: How do you manage patients who experience a progressive hyperopic shift after RK?
Drs. Vaidya and Majmudar: Initial management strategies include glasses and contact lenses, often specialty lenses such as rigid gas permeable and scleral contact lenses. In the absence of lenticular changes (ie, cataract), a patient with a small, stable hyperopic shift may be a candidate for laser vision correction—usually PRK. These enhancements are often technically challenging to execute, and the outcome is slightly unpredictable. Extensive preoperative counseling is therefore required.
A determining factor in management is whether the patient is experiencing diurnal fluctuations in vision. These can be especially bothersome to individuals who otherwise have good BSCVA; they can see well but must rotate through multiple pairs of glasses over the course of the day. Patients who experience significant diurnal fluctuations may not be good candidates for a refractive enhancement and may require penetrating keratoplasty or deep anterior lamellar keratoplasty.
CRST: How do you manage patients who have developed ectasia after RK?
Drs. Vaidya and Majmudar: We treat it similarly to other forms of ectasia such as keratoconus or post-LASIK ectasia. If the patient has good BSCVA or can wear specialty contact lenses and achieve acceptable visual acuity, CXL can be considered to halt ectatic progression. If the patient has not maintained good BSCVA, is intolerant of contact lenses, or experiences significant diurnal fluctuations, corneal transplantation may be required.
CRST: How do you manage patients who have corneal scars or Salzmann nodular degeneration that requires surgery after RK?
Drs. Vaidya and Majmudar: Ocular surface surgery such as the removal of Salzmann nodules must be performed with great care in these patients. Most RK incisions are not full thickness, but it is important to recognize and prepare for possible perforation during any manipulation of the cornea. Sutures should be on hand.
CRST: Would you consider a phakic IOL for an RK patient with continuous hyperopia? If so, please describe your approach and/or share an example.
Drs. Vaidya and Majmudar: Unfortunately, phakic IOL implantation is not approved for hyperopia in the United States, where we practice. Elsewhere, hyperopic phakic IOLs may be a viable bridge to cataract surgery. RK patients, however, are likely 50 years of age or older and at least prepresbyopic, which would reduce the advantage of preserving the natural lens. In the appropriate patient, RLE is probably a better strategy.
CRST: Would you consider RLE in a patient with a history of RK?
Drs. Vaidya and Majmudar: RLE can be a reasonable strategy for this population, but it can be difficult to achieve the desired refractive outcome. IOL calculations in eyes with irregular corneas are often challenging. Multiple imaging modalities must be used to evaluate corneal curvature and increase the chances of refractive success. Online tools such as the ASCRS postrefractive IOL calculator can be extremely helpful. Intraoperative wavefront aberrometry can also be useful, although readings may be inaccurate if there is significant corneal irregularity. The LAL has become the IOL of choice for some surgeons because it allows postoperative adjustment of the refractive endpoint and astigmatic correction. The IC-8 Apthera IOL can also work well in eyes with significant corneal irregularity and/or irregular astigmatism due to RK incisions.
CRST: How often do you see patients who underwent LASIK in the 1990s and early 2000s when technology was less advanced, and what are some of their common complaints?
Drs. Vaidya and Majmudar: Early excimer lasers were broad-beam rather than flying-spot, and the ablation energies or fluences and patterns were primitive compared to those of current systems. Most patients from the first generation of laser vision correction, however, do not have progressive visual complaints. If they have a decentered ablation or other irregularity that led to HOAs, these individuals certainly may benefit from a corrective, modern topography-guided excimer laser treatment. US surgeons do not have access to fully transepithelial topography-guided excimer laser ablation. In our opinion, this is a tremendous unmet need. The treatment of early LASIK patients has therefore been more successful outside the United States in our estimation.
CRST: How do you approach cataract surgery in patients with a history of RK or early LASIK?
Drs. Vaidya and Majmudar: Our approach to both subsets of patients is similar. A careful evaluation of the ocular surface is critical. In the past, it was thought that patients’ pre-LASIK refractive data were important to surgical planning, but evidence suggests that the historical method of IOL calculation is less accurate than modern IOL formulas. The detection of HOAs may guide IOL selection.
CRST: Would you implant a multifocal IOL in a patient who has a history of RK or early LASIK?
Drs. Vaidya and Majmudar: In our experience, the corneas of post-RK eyes are generally too irregular to support a multifocal or EDOF IOL. If the patient has a history of successful monovision (either with the RK procedure or contact lenses), this strategy may provide them with a degree of spectacle independence.
For patients who underwent an early LASIK procedure, an EDOF IOL may be considered if the ablation is well centered and the severity of HOAs is low. Diffractive multifocal IOLs offer less predictable results but may be an option for select individuals.
Obtaining informed consent is critical. Patients must be aware that an IOL exchange may be necessary.
CRST: Would you implant a small-aperture IOL in a patient who has a history of RK or early LASIK?
Drs. Vaidya and Majmudar: In general, individuals with significant irregular astigmatism can benefit from small-aperture optics, and these patients are no exception.
Case Example
A 67-year-old man presented with decreased visual acuity and increasing glare. He had undergone myopic LASIK 20 years earlier.
On examination, the patient’s uncorrected distance visual acuity was 20/25 OU, and his uncorrected near visual acuity was J10 OD and J12 OS. His distance visual acuity did not improve significantly with a mild hyperopic refraction in either eye. Biometry is shown in Figure 1.

Figure 1. Biometry of the right (A) and left (B) eyes.
The physical examination was notable for an intact LASIK flap and a trace to 1+ nuclear sclerotic cataract in each eye. Topography showed a well-centered ablation pattern and relatively regular astigmatism in each eye (Figure 2).
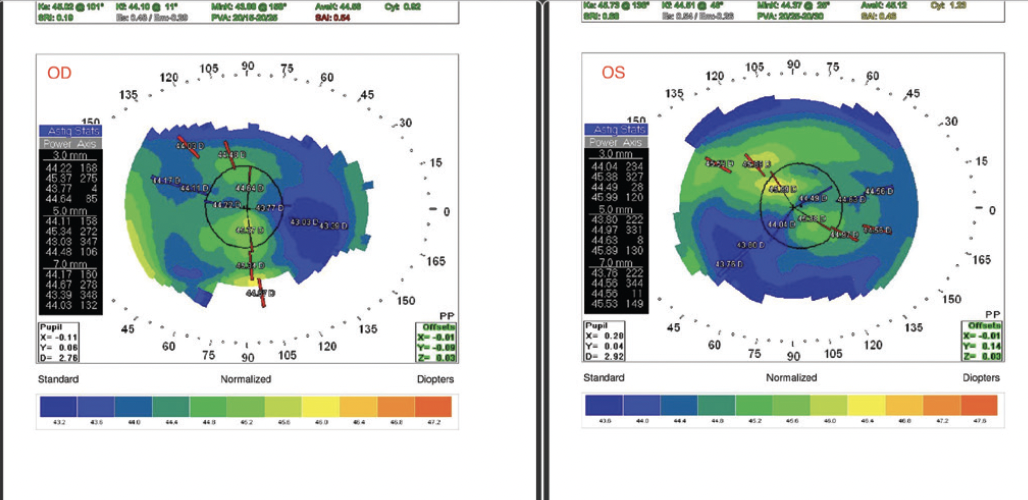
Figure 2. Preoperative topography of both eyes.
After a thorough discussion, the patient elected to undergo cataract surgery. He was extremely motivated to reduce his dependence on spectacles but willing to wear reading glasses for some near tasks.
A toric extended depth of focus IOL (AcrySof IQ Vivity, Alcon) was selected. Biometry measurements with the Lenstar (Haag-Streit) and topography readings were obtained, and the relevant data were entered into the ASCRS postrefractive IOL calculator. After reviewing results with the various formulas represented in the calculator (Figure 3), a 19.50 D IOL was chosen to achieve a refractive outcome close to plano in each eye.
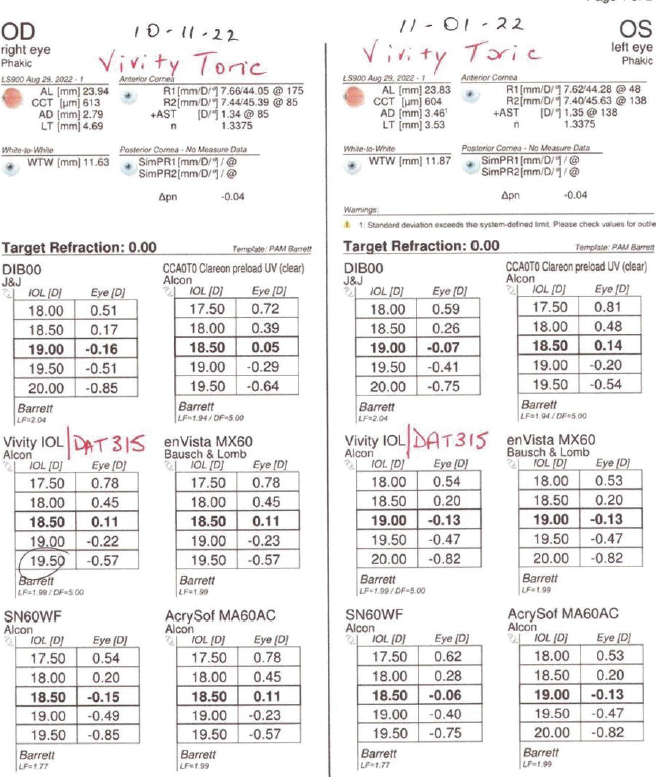
Figure 3. IOL power calculation with various formulas.
Intraoperative aberrometry with the ORA System (Alcon) was performed to compare with the IOL calculations and confirm proper toric IOL alignment. The ORA-predicted outcome correlated well with the preoperative assessment for the left but not the right eye. A 19.00 D Vivity (model DAT315) IOL was implanted in the right eye, and a 19.50 D Vivity (model DAT415) IOL was implanted in the left.
One month after surgery on the second eye, the patient’s uncorrected distance visual acuity was 20/25+ OU, and his uncorrected near visual acuity was J2 OD and J2 to J3 OS with a minimal myopic spherical refraction. The patient was highly satisfied.
CRST: Would you implant an LAL in a patient who has a history of RK or early LASIK?
Drs. Vaidya and Majmudar: The LAL really shines in these patient populations. As noted earlier, given the challenges of IOL selection, the ability to make adjustments postoperatively and accurately to treat astigmatism makes the LAL a great option for these individuals. Additionally, the LAL is essentially a monofocal IOL, so it is not subject to the same limitations as a diffractive IOL.
CRST: What is your preferred IOL power calculation for patients who underwent RK or early LASIK?
Drs. Vaidya and Majmudar: The keys to success are using multiple tools and looking for congruence. Obtaining multiple sets of measurements with different devices and looking for consistent outcomes can help reduce variability. Modalities such as OCT-guided corneal power calculations and the ASCRS postrefractive online calculator can be extremely helpful as well.
CRST: If you use intraoperative aberrometry, what specific benefits does the technology offer for patients who underwent RK or early LASIK?
Drs. Vaidya and Majmudar: Intraoperative aberrometry is valuable in these situations because it allows real-time analysis of the eye’s refractive state before an IOL is implanted. We regularly perform intraoperative aberrometry in both of these patient populations and compare the results with preoperative measurements. Looking for consistency across all acquired data is the key to success. Intraoperative aberrometry provides an additional data point to help reduce variability in refractive outcomes. A word of caution: Intraoperative aberrometry may not yield accurate results in eyes with highly irregular corneas, so the surgeon’s judgment is critical.
CRST: Do you consider a two-step procedure with a laser ablation followed by cataract surgery for individuals who have irregular corneal astigmatism as a result of their previous refractive procedure?
Drs. Vaidya and Majmudar: It is certainly preferable to correct any surface irregularity, if possible, before undertaking cataract surgery.
CRST: How would you perform a glaucoma assessment in eyes that underwent RK or early LASIK?
Drs. Vaidya and Majmudar: It can be difficult to obtain accurate IOP readings in these eyes. Numerous studies have evaluated the effect of refractive surgery on applanation tonometry. Eyes with thin corneas have artificially low IOP measurements with this test. It is important to pay close attention to the appearance of the optic nerve and monitor it for progressive cupping. Serial visual field testing should also be performed and evaluated closely for early glaucomatous defects.
CRST: What tips do you have for treating OSD and DED in eyes that underwent RK or early LASIK?
Drs. Vaidya and Majmudar: Treating the ocular surface is important because it removes one variable from a complex situation. We routinely treat patients with copious amounts of artificial tears, topical cyclosporine or lifitegrast, punctal plugs, and serum tears. Some patients may benefit from intense pulsed light treatment and other technologies as well.




