CASE PRESENTATION
A 24-year-old man presents 2 years after undergoing CXL to request additional intervention to correct his refractive error. The patient has attempted to wear contact lenses several times without success.
Upon presentation, his UCVA is 20/70 OD and 20/40 OS. His BCVA is 20/25 OU with a manifest refraction of -4.25 +3.00 x 153º OD and -3.50 +4.50 x 035º OS. The patient’s refraction has been stable for the past 6 months.
Central corneal thickness is 509 µm OU. The corneal and retinal exams are normal. Imaging with the iTrace (Tracey Technologies) shows a clear lens in the right eye and slight lenticular opacification in the left eye (Figures 1 and 2).
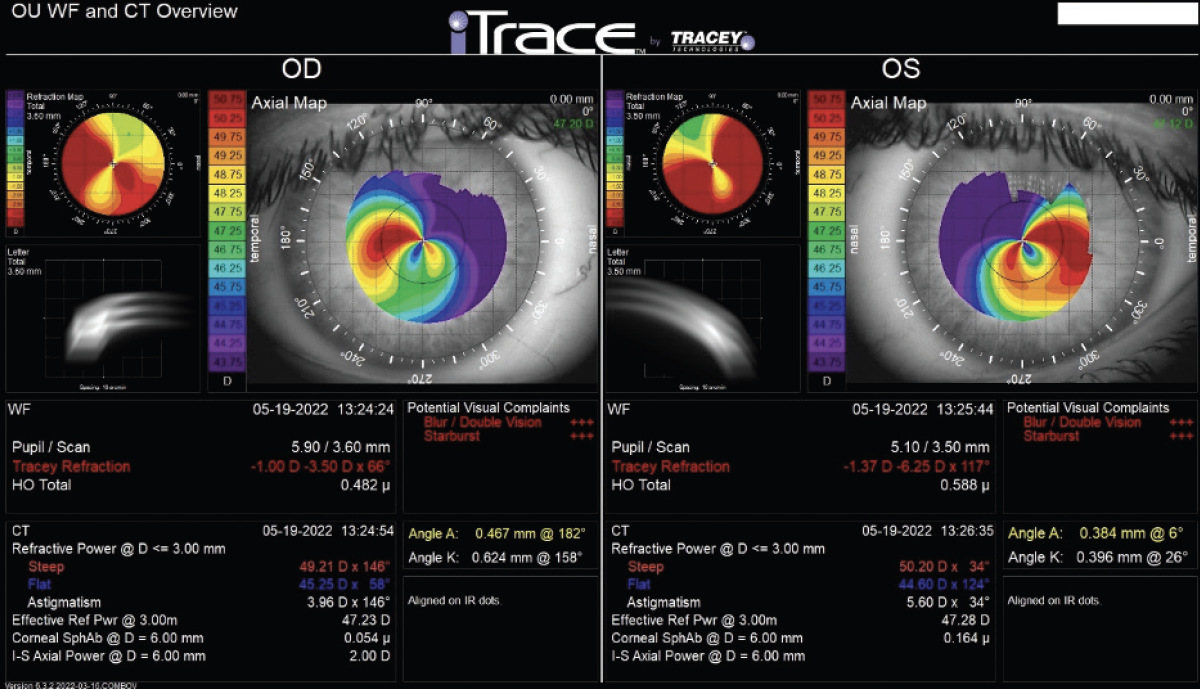
Figure 1. iTrace analysis of the right and left eyes. Wavefront and computed tomography overview.
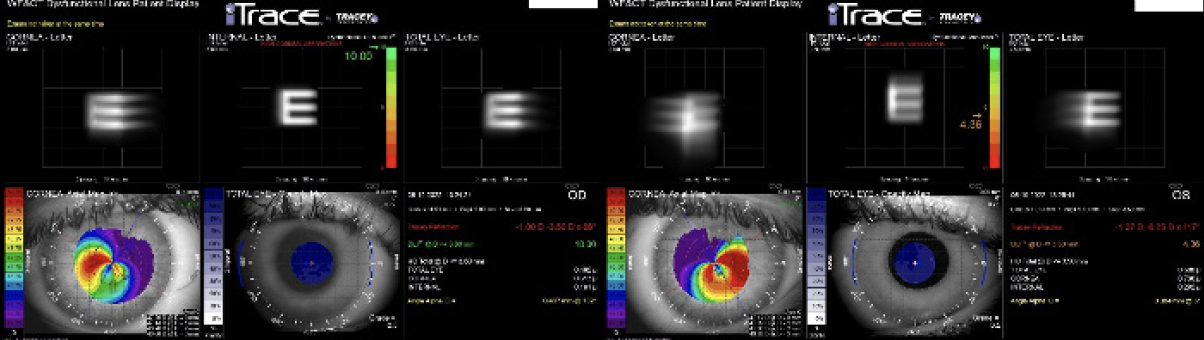
Figure 2. The dysfunctional lens index was 10.00 OD and 4.36 OS.
How would you proceed?
—Case prepared by Karl G. Stonecipher, MD

A. JOHN KANELLOPOULOS, MD
Successful CXL thickens the cone epithelium to a normal or nearly normal level.1 This finding can be used to confirm disease stability after CXL, as can the CXL line visible with anterior segment OCT; I use the Avanti (Visionix). The stability of the cone can be monitored over time with epithelial maps, which I find are most easily obtained with epithelial OCT (Figure 3).
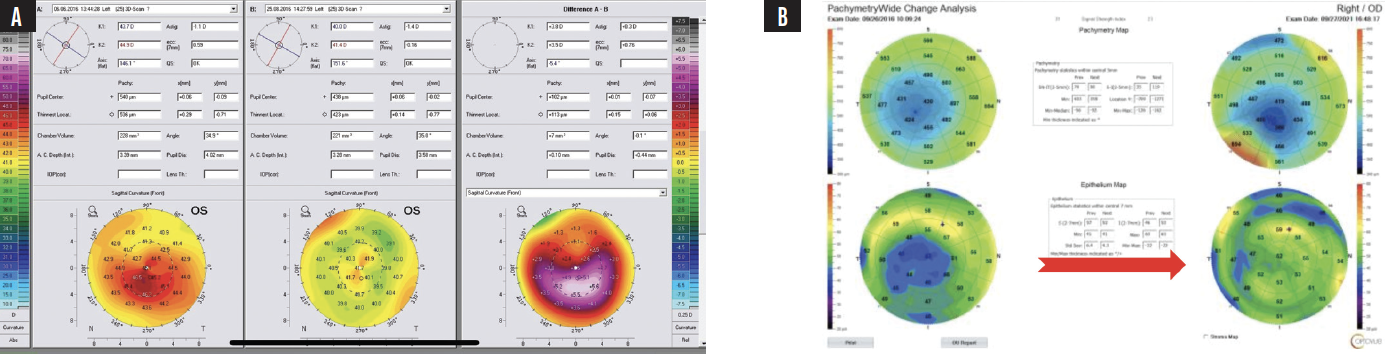
Figure 3. An example of drastic total corneal power reshaping following CXL using the Athens protocol. The difference map on the right (A) confirms the accuracy of surface reshaping treatment with the WaveLight EX500 excimer laser (Alcon). The preoperative (left) and postoperative (right) corneal and epithelial maps demonstrate epithelial remodeling (B). The initially thin epithelium over the center of the cone becomes more normal and thicker after surface reshaping with the excimer laser and CXL. The epithelial change can be erroneously identified as steepening and progressive ectasia. This case example shows the potential utility of therapeutic excimer laser treatment and the clinical importance of the correlation between epithelial maps and topographic or tomographic assessments of corneal curvature.
Courtesy of A. John Kanellopoulos, MD
Eye rubbing, including while the patient is asleep, is a factor to consider because the patient is in his mid-20s and potentially at increased risk of ectasia. I ask patients with keratoconus to record themselves while sleeping and typically find that the more affected eye is positioned on their knuckles while they sleep. The use of a thick face mask can help these individuals in the long term. (Watch the video below for more on the use of face masks.)
Assuming the patient’s cornea and refraction are stable, he is part of a lucky minority of individuals with keratoconus. The cone in each eye is central, so the corneal astigmatic changes appear to be almost regular oblique astigmatism. The only downside for this subset of patients is that the cone is usually thin. Fortunately, the thickness of the patient’s cones in this case is greater than 500 µm, which gives him multiple options for how to proceed.
Option No. 1: Surface ablation. This form of laser vision correction could greatly improve the patient’s refractive error. Given the regularity and central location of the cone, even a standard wavefront-optimized ablation could be effective. Because of the amount of astigmatism present, in my opinion, two major benefits of performing surface ablation are the accuracy of eye tracking and the ability to adjust cyclorotation. The effective astigmatic correction decreases by almost 8% for every 2º off the real axis treatment is.
Option No. 2: A phakic IOL. Most eyes with keratoconus have deep anterior chambers, so the implantation of an EVO ICL (STAAR Surgical) would be an excellent alternative that would leave multiple options open to him if he experiences future corneal changes. This could be an advantage because keratoconus can later progress, particularly if patients do not cease rubbing their eyes. Others demonstrate progressive flattening of the cone over time.2 In either scenario, future refractive intervention may be required if contact lens wear is intolerable. Another benefit of an ICL is that postoperative recovery is more rapid and side effects are milder compared with the other two options for treatment I discuss.
Careful axial alignment with either intraoperative wavefront guidance or intraoperative imaging can be helpful during surgery.
Option No. 3: Refractive lens exchange (RLE). This procedure would be my last choice because of the patient’s age, corneal thickness, and refractive error.
Surface ablation would be my preference because of the corneal thickness and the fact that a topography-guided ablation could reshape the cornea so that it is symmetric to the corneal vertex (ie, line of sight). A ray-tracing customized ablation might produce even better results.3

IVAN MAC, MD, MBA
The patient’s situation is rendered more complex by early-stage dysfunctional lens syndrome. Imaging with the iTrace demonstrates mild compromise of the internal optics of each eye. He has options, but each entails a tradeoff.
Option No. 1: Surface ablation. Topography-guided PRK is one possibility. The steep axis on the refraction matches well with the steep axis identified with the iTrace, and the cylinder correction is within the range of treatment approved in the United States, where I practice. That said, PRK would remove a lot of tissue. A central corneal thickness of 509 µm OU is adequate, but pachymetry would be repeated to ensure that the readings are accurate.
The patient should be counseled that an enhancement may be required, informed that he has early nuclear sclerosis, and educated on the implications of the diagnosis.
Option No. 2: A phakic IOL. The EVO ICL can treat up to 4.00 D of astigmatism. Implantation would leave the left eye with minimal residual cylinder, would be the least invasive of the three options I discuss, and would allow a laser enhancement procedure to be performed in the future if the refraction changes slightly.
Proper intraoperative alignment of the ICL is critical to maximizing the cylinder correction. A digital marking system such as the Verion Image Guided System (Alcon) or Callisto eye (Carl Zeiss Meditec) could be helpful in this case. The anterior chamber depth of each eye would be measured to ensure it is adequate for the ICL.
Again, the patient would be informed of his early nuclear sclerosis and his possible future need for an enhancement.
Option No. 3: RLE. This alternative is the least desirable for a 22-year-old patient. An IOL with diffractive optics is contraindicated because of the high levels of negative coma induced by the corneal changes from keratoconus. A toric extended depth of focus IOL could provide an adequate range of vision. The patient’s near vision, however, would not be as good as what he currently has, and he might experience some dysphotopsias at night.
An alternative is a small-aperture lens such as the IC-8 Apthera (AcuFocus). The Apthera elongates the depth of focus. It could deliver high-quality vision with minimal dysphotopsias but at the cost of some near vision and the potential dimming of vision in low light.
If the corneal endothelial cell count and anterior chamber depth are within appropriate limits, my preference would be to implant an EVO ICL. A fourth option is to place Intacs (CorneaGen) to decrease the cylinder and potentially reduce higher-order aberrations.

NEDA NIKPOOR, MD
When patients who have keratoconus are interested in vision correction, I recommend they undergo CXL first and wait until corneal stability is achieved before proceeding with a vision correction procedure. In most cases, eyes are relatively stable 1 to 2 years after CXL.
I would like to know the patient’s uncorrected and corrected distance and near visual acuities. He would also be examined at the slit lamp because I question the validity of the iTrace dysfunctional lens index for the left eye. The patient is only 22 years old. If no cataract is present on examination, then I would assess his BCVA with spectacles monocularly. If a high-quality level of vision with no more than 4.00 D of cylinder correction at the spectacle plane can be achieved and good distance-corrected near vision is maintained, I would repeat measurements with the iTrace and potentially disregard the readings if they do not fit with other findings.
Option No. 1: Surface ablation. If the patient’s BCVA is unsatisfactory, I would discuss topography-guided PRK with him. The procedure could improve his higher-order aberrations and quality of vision and reduce his irregular astigmatism and refractive error. Multiple sessions over the course of several months might be required. Gatinel and others have shown that PRK is safe in keratoconic eyes if the level of correction required is low to moderate and corneal thickness is adequate.4 I would nevertheless discuss with the patient the rare possibility that surgery could induce keratoconic progression or ectasia.
Option No. 2: A phakic IOL. If the patient is satisfied with his BCVA, I would discuss a Visian Toric ICL (STAAR Surgical) with him. I favor this procedure for individuals with keratoconus because it preserves their corneas for future surgery such as a PRK enhancement.
Option No. 3: RLE. I would avoid RLE in this young myopic patient until he has no other options for vision correction.
Whichever procedure is ultimately pursued, it would be essential to counsel the patient that the goal of surgery is functional (ie, good enough) rather than perfect vision. Before proceeding, I would explain the inherent unpredictability of refractive outcomes, the increased chance that he will need an enhancement, and the refractive unpredictability of an enhancement owing to his keratoconus. If he is comfortable with the tradeoffs and prefers functional uncorrected vision over the superior quality of vision that can be achieved with rigid contact lenses, I would feel comfortable proceeding with surgery and would expect him to do well.

WHAT I DID: KARL G. STONECIPHER, MD
The panelists outline the pluses and minuses of the three main treatment options for the patient. At the time this article was written, I had counseled him that the most reversible procedure would be the implantation of an EVO ICL, but he had not yet decided on an intervention.
Editor’s note: The use of a partial in refraction excimer laser surface ablation combined with CXL for progressive keratoconus is off label.
1. Kanellopoulos AJ, Vingopoulos F, Sideri AM. Long-term stability with the Athens protocol (topography-guided partial PRK combined with cross-linking) in pediatric patients with keratoconus. Cornea. 2019;38(8):1049-1057.
2. Kanellopoulos AJ. Ten-year outcomes of progressive keratoconus management with the Athens protocol (topography-guided partial-refraction PRK combined with CXL). J Refract Surg. 2019;35(8):478-483.
3. Kanellopoulos AJ. Keratoconus management with customized photorefractive keratectomy by artificial intelligence ray-tracing optimization combined with higher fluence corneal crosslinking: the ray-tracing Athens protocol. Cornea. 2021;40(9):1181-1187.
4. Guedj M, Saad A, Audureau E, Gatinel D. Photorefractive keratectomy in patients with suspected keratoconus: five-year follow-up. J Cataract Refract Surg. 2013;39(1):66-73.