Ultrathin DSAEK
Frederic Aissani, MD

A 73-year-old woman presented with Fuchs endothelial dystrophy in both eyes. The patient reported progressive vision loss in her right eye and expressed a desire to improve vision in this eye. The patient was pseudophakic. Her visual acuity was 10/100 OD, and central corneal thickness was 690 µm OD. The left eye was amblyopic. Ultrathin Descemet stripping automated endothelial keratoplasty (DSAEK) was planned.
A temporal 4-mm scleral incision and two paracenteses were made at the 6 and 12 clock positions. An anterior chamber maintainer was placed. A 2.85-mm angle blade was used to create an incision in the temporal cornea under an infusion of balanced salt solution (Figure 1). The surface of the cornea was stained 8.5 mm circumferentially. Trypan blue dye (VisionBlue, Dutch Ophthalmic USA) was instilled into the anterior chamber. The Descemet membrane was removed using a scraper. This tissue was then withdrawn from the anterior chamber using capsulorhexis forceps and placed on the corneal surface (Figure 2).
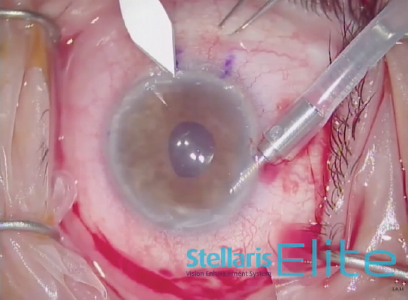
Figure 1. A 2.85-mm angle blade is used to create an incision in the temporal cornea under an infusion of balanced salt solution.
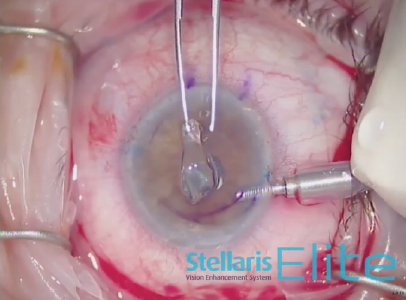
Figure 2. Capsulorhexis forceps are used to withdraw the Descemet membrane from the anterior chamber.
An 8.5-mm endothelial graft was prepared and inserted in the anterior chamber using a Busin glide and 23-gauge forceps (Figure 3). Immediately thereafter, the infusion line was switched off, and the anterior chamber maintainer was removed. The four sideport incisions were closed. Next, a bubble of filtered air was injected under the graft, and it was centered under the recipient stroma as much as possible (Figure 4). Once all sutures were in place, the air bubble was left in the anterior chamber for 20 minutes while the stromal interface was dryed to promote better adhesion (Figure 5).
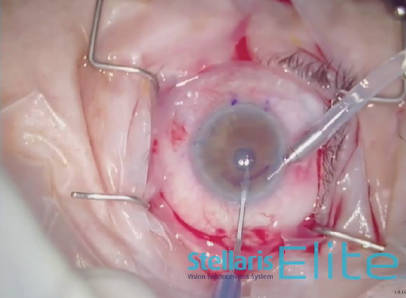
Figure 3. A Busin glide and 23-gauge forceps areused to insert an 8.5-mm endothelial graft into the anterior chamber.
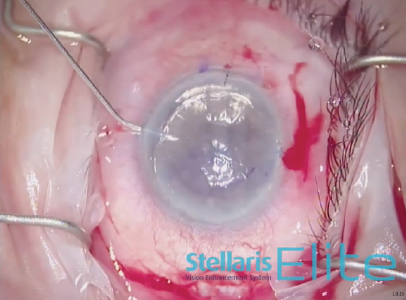
Figure 4. A bubble of filtered air is injected under the graft.
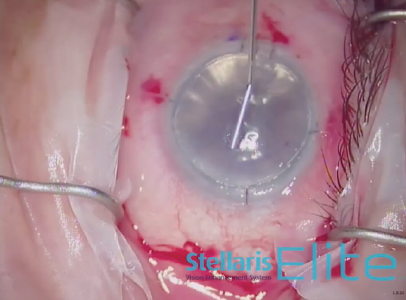
Figure 5. Drying the interface promotes better adhesion.
See video below to watch the case.
Combined Surgery After Longstanding Eyelash in Eye
Ozana Moraru, MD

A 60-year-old patient presented with decreased visual acuity due to a longstanding eyelash in the eye. The presence of the eyelash had caused the development diffuse corneal edema, stromal haze, dense Descemet folds, and neovessels in the inferior part of the cornea.
Ultrasound biomicroscopy and OCT imaging showed that the eyelash was in the anterior chamber attached to the endothelium. An advanced cataract was also present. Combined cataract extraction and DSAEK were planned.
Three days before surgery, the patient received a subconjunctival injection of bevacizcumab (Avastin, Genentech) inferonasally to address the neovessels. Due to poor visualization before and after epithelial removal and staining (Figure 6), phacoemulsification, rather than Descemet removal under air, was performed first to avoid constricting the pupil during the Descemetorhexis. The eyelash was removed easily under an OVD (Figure 7). After phacoemulsification, the Descemet membrane was removed under air. The nasal paracentesis was then enlarged, and a hydrophobic posterior chamber IOL was injected under balanced salt solution (Figure 8). The delayed timing of IOL implantation was because the large incision could have made Descemet removal under air difficult. Acetylcholine (Miochol-E, Bausch + Lomb) was instilled, a peripheral iridectomy was performed at the 6 clock position, and standard DSAEK was performed (Figure 9).
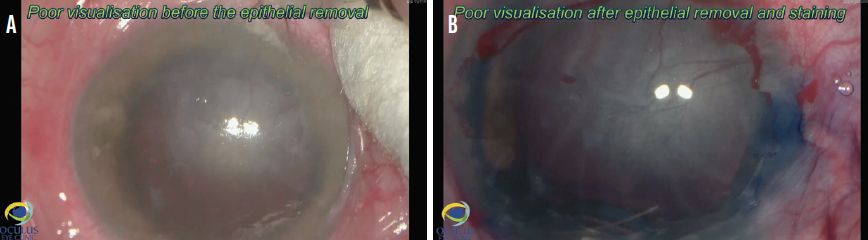
Figure 6. Poor visualization before epithelial removal and staining (A) and after epithelial removal and staining (B).
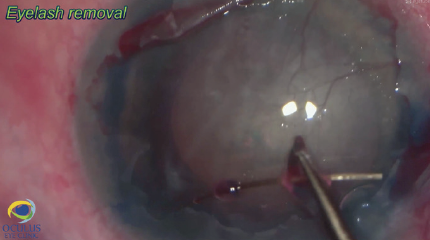
Figure 7. The eyelash is removed under an OVD.
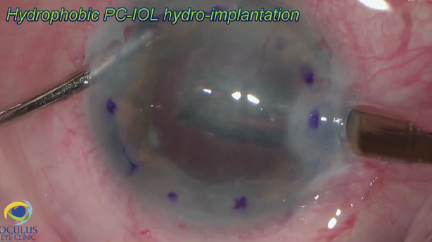
Figure 8. The nasal paracentesis is enlarged, and a hydrophobic posterior chamber IOL is injected under balanced salt solution.
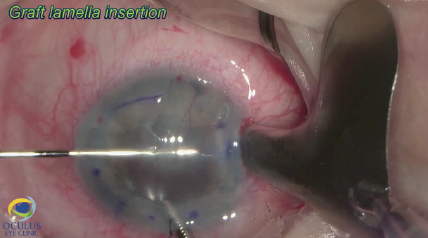
Figure 9. DSAEK is performed.
See video below to watch the case.
Penetrating Keratoplasty and IOL Exchange
Zeba A. Syed, MD

A 68-year-old woman presented with corneal scarring, corneal edema, and an anterior chamber IOL. Penetrating keratoplasty and an IOL exchange were planned.
To start the case, the limbus was marked at the 6 and 12 clock positions, and the sclera was marked 2.5 mm behind each of these limbal marks. A Flieringa ring was sutured in place, and the cornea was trephinated and cut off using right- and left-handed scissors (Figure 10). The anterior chamber IOL was found to be scarred into the iris. Instead of pulling the lens to remove it, the haptic was cut at its point of adhesion to the iris, and the IOL was safely removed from the eye (Figure 11). A limited open-sky anterior vitrectomy was performed to create space behind the iris for a new one-piece IOL (CZ70BD, Alcon). After the vitrectomy, a 5-0 polypropylene suture (Prolene, Ethicon) was threaded through each of the eyelets, and low-temperature cautery was used to create a large bulb at each end of the lens to prevent slippage through the eyelets (Figure 12). Then, one at a time, the needle associated with each of the sutures was pulled under the iris and out through the premarked area on the sclera to position the lens (Figure 13).
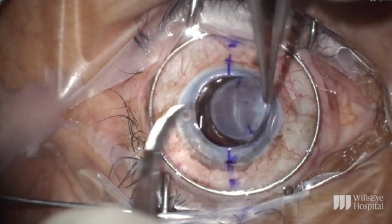
Figure 10. A Flieringa ring is sutured in place, and the cornea is trephinated and cut off using right- and left-handed scissors.
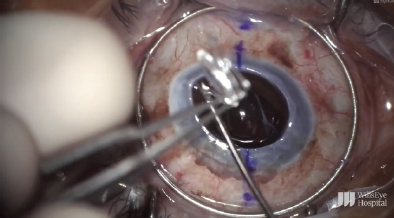
Figure 11. The haptic is cut at its point of adhesion to the iris, and the IOL is safely removed from the eye.
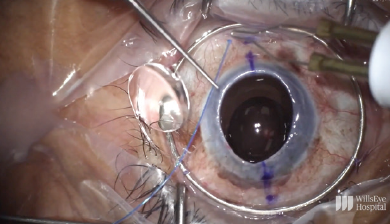
Figure 12. A 5-0 polypropylene suture is threaded through an eyelet, and low-temperature cautery is used to create a large bulb at each end of the lens to prevent slippage through the eyelet.
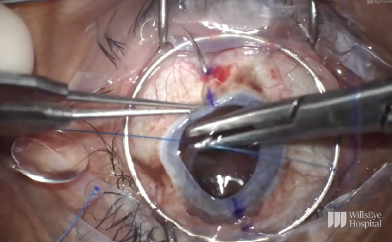
Figure 13. The needle associated with one of the sutures is pulled under the iris and out through the premarked area on the sclera to position the lens.
The graft was sutured into place (Figure 14). After placement of the first eight sutures, the eye was pressurized, and the appropriate tension for the IOL was determined.
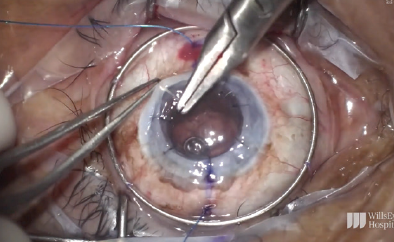
Figure 14. The graft is sutured into place.
The polypropylene sutures were cut to the appropriate length, and low-temperature cautery was used to create a flange that was buried subconjunctivally on both sides. The remaining eight sutures were then placed, the 16 sutures were rotated, and the case was completed.
See video below to watch the case.