
A new paradigm for glaucoma management has emerged: interventional glaucoma. Specialists who have adopted an interventional glaucoma mindset appreciate how poor adherence to prescribed medical therapy influences the disease course, how glaucoma progression affects patients’ quality of life, and how important early intervention—well before significant damage to the optic nerve occurs or functional vision is lost—is to successful long-term care. Integral to this paradigm shift is the introduction of technologies that can lower IOP and reduce the burden of topical glaucoma therapy while maintaining a high level of intra- and postoperative safety. These technologies include selective laser trabeculoplasty, drug delivery devices, trabecular bypass stents, canaloplasty, goniotomy/trabeculotomy, cilioablative lasers, and subconjunctival bypass devices. Earlier intervention for glaucoma—often in combination with cataract surgery—can preserve a heathy retinal nerve fiber layer and ganglion cell complex (GCC).
When approaching cataract surgery in patients with glaucoma, ophthalmologists often focus on the glaucoma or the cataracts, either forgetting to offer a glaucoma procedure or not thinking it important to offer patients premium lens options. Surgery for this patient population requires unique considerations, but the goal of maximizing quality of life and satisfaction is the same whether or not a cataract patient has glaucoma.
THE BENEFITS OF CATARACT SURGERY
Cataract surgery itself can benefit patients with glaucoma by reducing IOP and potentially improving contrast sensitivity through the extraction of the cloudy crystalline lens. In order to maximize the chances of decreasing the burden of topical medical therapy and reducing IOP, I generally offer to combine cataract surgery with MIGS for these patients. Not only can reducing the drop burden through combining these two procedures improve patient adherence, but it can also decrease these individuals’ cumulative probability of undergoing incisional glaucoma surgery, as found in the HORIZON trial of the Hydrus Microstent (Ivantis).1
Reducing the drop burden can also help to maintain a healthier ocular surface, which can, in turn, increase quality of vision (discussed later in more detail). In a phase 3 pivotal trial of the iStent Inject (Glaukos), patients who received this device at the time of cataract surgery reported a more significant improvement in their ability to drive at night, general vision, and ocular surface symptoms than those who underwent cataract surgery alone.2
IOL SELECTION
I often hear colleagues say that they would not implant a premium IOL in a patient with glaucoma. I think, however, this is too broad a dictum. It is important to think about the quality of vision in these patients, and that encompasses considering the benefits, risks, and safety profiles of each lens category.
Monofocal IOLs. I tend to choose a monofocal lens, preferably one with an aberration-free optic such as the enVista (Bausch + Lomb), for patients with pseudoexfoliative glaucoma and those with significant glaucomatous damage. The IOL power is the same from center to edge, and these lenses are therefore more forgiving of tilt and decentration. A lens with zero aberrations also leaves behind slightly positive spherical aberration, which can allow slightly more depth of focus. I also generally recommend a monofocal IOL if a patient has sustained significant GCC damage, glaucoma has been or is progressing, changes are evident in the central 10º of visual field, or significant cupping of the optic nerve is evident. Other suitable lens options in these four instances include accommodating and extended depth of focus (EDOF) IOLs (discussed later).
Multifocal and trifocal IOLs. Contrast sensitivity may be a marker of early damage from glaucoma, and this disease has been shown to affect contrast sensitivity to a greater degree than visual acuity.3-6 Decreased contrast sensitivity can cause visual symptoms even when visual acuity is normal. Because light splits as it passes through a multifocal lens, less light reaches the retina, and contrast sensitivity is reduced. The use of multifocal IOLs for patients with glaucoma must therefore be approached with caution. Newer-generation trifocals, however, typically offer less loss of contrast sensitivity than older-generation multifocal IOLs.
If a patient has early (ie, mild) glaucoma that has been stable for more than 1 year, the GCC is healthy overall, and there is no loss of function (especially near the central 10º of visual field), I feel comfortable offering a trifocal IOL (Figure 1). Despite a normal 24-2 at baseline, a 10-2 visual field test is performed to confirm no loss of sensitivity in the central 10º. Patients with secondary glaucoma from diabetes are poor candidates for multifocal IOLs because of potential retinal issues, including cystoid macular edema.
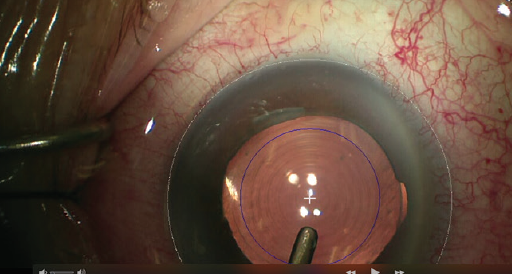
Figure 1. In this combined cataract surgery and MIGS procedure, an iStent was placed and an AcrySof IQ PanOptix trifocal IOL (Alcon) was implanted.
EDOF and accommodating IOLs. These lens types provide a higher degree of contrast and have better modulation transfer function (MTF) curves compared to multifocal lenses. Accommodating and EDOF IOLs are therefore options for some patients with moderate or even advanced glaucoma. The aspheric, aberration-free optic of the Crystalens (Bausch + Lomb) is more forgiving of decentration and tilt than a multifocal IOL, and the optic has the same characteristics as a standard monofocal lens. It therefore may be suitable for implantation in an eye with glaucoma of any severity. Other options include the Tecnis Eyhance (Johnson & Johnson Vision), a nonpremium monofocal lens that has properties that can provide some patients with a slightly extended range of vision; the AcrySof IQ Vivity IOL (Alcon), an EDOF lens that provides fairly high contrast and a minimal decrease compared to its standard monofocal relative and achieves this range of vision with two smooth surface transition elements that work simultaneously to stretch and shift light without splitting it7; and the Tecnis Symfony IOL (Johnson & Johnson Vision), which elongates focus instead of splitting light into distinct focal points, resulting in an increased depth of field. The echellete design of the Symfony allows a range of continuous vision while maintaining overall high image contrast.8 A recent study comparing a monofocal, a trifocal, and the Symfony IOL demonstrated similar contrast sensitivity with all three types of IOLs at an average of 60 months after implantation. All three IOL types also had similar MTF curves.9
Another option, once approved in the United States, is the IC-8 IOL (AcuFocus). This lens is designed to provide 3.00 D of EDOF to tolerate up to 1.00 D of deviation from the targeted manifest refraction spherical equivalent. The IC-8 can accommodate as much as 1.50 D of corneal astigmatism and reduce the effects of both lower- and higher-order aberrations such as coma through a small-aperture effect.10 An IC-8, however, may be a poor choice for patients with significant visual field or GCC loss (Figure 2) because it may further decrease visual field and potentially contrast sensitivity.
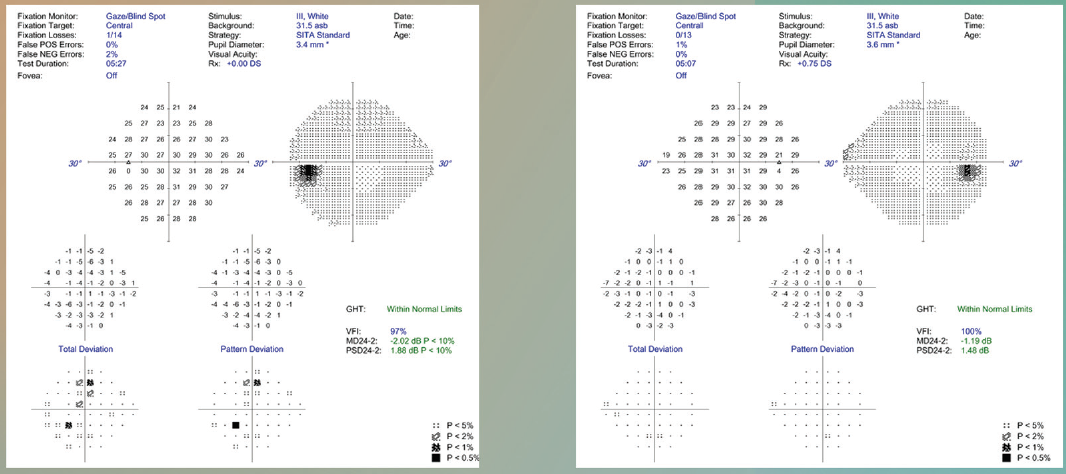
Figure 2. This patient had overall healthy visual fields but an unhealthy retinal nerve fiber layer and GCC.
I should emphasize that patients who have experienced severe glaucomatous damage and loss of fixation may not appreciate the benefits of extended range of vision. The cost-benefit analysis may therefore be unfavorable to the implantation of accommodating and EDOF lenses.
Toric IOLs. It is important to maximize the quality of light entering an eye with glaucoma because this disease reduces patients’ contrast sensitivity. Even low amounts of cylinder can negatively affect the MTF. I find the iTrace (Tracey Technologies) invaluable for evaluating higher-order aberrations in the cornea and crystalline lens and for educating patients about the quality of vision they could achieve with astigmatic correction, whether with a toric IOL or relaxing incisions. The correction of even a low amount of astigmatism can have a significant impact on patients’ quality of vision (Figure 3). A monofocal toric IOL is safe even for patients with more advanced glaucoma.
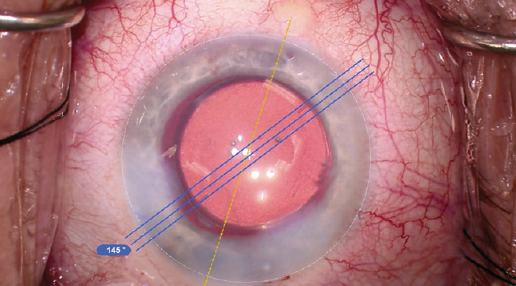
Figure 3. In this combined cataract surgery and MIGS procedure, a Kahook Dual Blade (New World Medical) was used and a toric IOL was implanted.
Adjustable IOLs. The Light Adjustable Lens (RxSight) is an option for some patients with glaucoma who undergo combined cataract surgery and MIGS. The sphere and cylinder can be titrated after the lens is implanted, allowing optimization of the refractive outcome once an eye achieves refractive stability postoperatively. I would consider this IOL for patients with glaucoma whose preoperative measurements are unstable, for those who have a history of filtering surgery but retain a good central 10º of visual field, for those in whom cataract and glaucoma surgery is expected to be complex, and for those with complex aberrated corneas.
THE OCULAR SURFACE
The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study found that up to 80% of patients have preexisting dry eye at the time of the cataract surgery consultation.11 It is reasonable to hypothesize that the percentage is higher among patients who are using topical glaucoma therapy because many studies have shown a correlation between increased ocular surface disease and the number of topical glaucoma medications.12
Ocular surface disease can negatively affect the accuracy of biometry measurements and thus IOL power calculations. I am therefore aggressive about optimizing the ocular surface before combined cataract surgery and MIGS. I routinely initiate treatment with antiinflammatory drops, including steroids (eg, loteprednol or fluorometholone acetate), a few weeks before surgery. I also prescribe an immunomodulator such as lifitegrast ophthalmic solution 5% (Xiidra, Novartis) or cyclosporine and consider treating meibomian gland dysfunction with a variety of treatments, including systemic antibiotics/oils, heat treatment/expression, intense pulsed light therapy, debridement, and topical sprays. I use a modern biometer, such as the IOLMaster 700 (Carl Zeiss Meditec), because it can decrease the acquisition time and therefore the chance of the tear film contributing to poor measurements.
When a patient has dry eye disease, I tend to offer selective laser trabeculoplasty to reduce the number of topical glaucoma drops required. I also consider replacing a topical agent for a period of time with a bimatoprost implant (Durysta, Allergan). This allows me to focus on the surface, especially before cataract surgery.
CONCLUSION
Cataract surgery presents a prime opportunity to optimize quality of life for patients with glaucoma. Technological and procedural advances have improved surgeons’ ability to manage both cataracts and glaucoma in a single procedure while maximizing patients’ quality of vision and their overall experience (for more on the patient experience, see Surgical and Procedural Advances).
Surgical and Procedural Advances
Performing combined cataract surgery and MIGS as an in-office procedure provides an opportunity to enhance the patient experience. Patients appreciate that they can engage with the same staff in the pre- and postoperative phases of their care, promoting continuity and accountability. Scheduling is also easier with in-office surgery because our coordinators can work with patients to select a convenient time for their procedures. We can also show patients the surgical suite during their preoperative consultation, which helps to reduce their stress level on the day of surgery. Lastly, we can more easily experiment with new technology because multiple approvals from administration are not required.
Other advances that can enhance the patient experience include the use of oral anesthesia in most cases and the incorporation of certain intraoperative technologies in glaucoma premium cases. Oral anesthesia typically reduces patients’ stress levels, improves their satisfaction, and promotes a more efficient flow of surgery. The miLoop (Carl Zeiss Meditec) can be useful for patients with dense cataracts or zonular weakness because it can decrease energy and allow a clearer view for the MIGS procedure. The use of a femtosecond laser or the Zepto (Centricity Vision) can also help with the creation of a centered capsulotomy in these cases. Incorporating technologies such as these can help surgeons optimize results, even in tough cases.
The surgeon’s job is to determine how to achieve a 20/happy result for each patient, and incorporating refractive cataract surgery into glaucoma care can help to achieve that in many cases. Combined cataract surgery and MIGS can address both glaucoma and refractive error in one procedure.
1. Ahmed IIK, Rhee DJ, Jones J, et al; HORIZON Investigators. Three-year findings of the HORIZON trial: a Schlemm canal microstent for pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology. 2021;128(6):857-865.
2. Samuelson TW, Singh IP, Williamson BK, et al. Quality of life in primary open-angle glaucoma and cataract: an analysis if VFQ-25 and OSDI from the iStent inject pivotal trial. Ophthalmology. 2021;229:220-229.
3. Richman J, Lorenzana LL, Lankaranian D, et al. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010;128(12):1576-1582.
4. Ross JE, Bron AJ, Clarke DD. Contrast sensitivity and visual disability in chronic simple glaucoma. Br J Ophthalmol. 1984;68(11):821-827.
5. Atkin A, Bodis-Wollner I, Wolkstein M, Moss A, Podos SM. Abnormalities of central contrast sensitivity in glaucoma. Am J Ophthalmol. 1979;88(2):205-211.
6. Sample PA, Juang PS, Weinreb RN. Isolating the effects of primary open-angle glaucoma on the contrast sensitivity function. Am J Ophthalmol. 1991;112(3):308-316.
7. Schwiegerling J, Carson D, Xu Z, et al. Optical simulation and bench data of a non-diffractive intraocular lens designed to extend depth of focus and minimize visual disturbances. Paper presented at: 37th Congress of the European Society of Cataract & Refractive Surgeons; September 14-18, 2019; Paris.
8. Takahashi M, Yamashiro C, Yoshimoto T, et al. Influence of extended depth of focus intraocular lenses on visual field sensitivity. PLOS one. doi.org/10.1371/journal.pone.0237728
9. Gundersen KG, Potvin R. Comparing visual acuity, low contrast acuity and contrast sensitivity after trifocal toric and extended depth of focus toric intraocular lens implantation. Clin Ophthalmol. 2020;14:1071-1078.
10. Ang RET, Picache GCS, Rivera MCR, Lopez LRL, Cruz EM. A comparative evaluation of visual, refractive, and patient-reported outcomes of three extended depth of focus (EDOF) intraocular lenses. Clin Ophthalmol. 2020;14:2339-2351.
11. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
12. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350-355.




