

The evolution of medical technology has led to extraordinary advances that benefit patients experiencing a broad range of health problems, and the role of clinicians has been crucial to ensuring the safety and efficacy of this evolution. Concerns, however, have been raised about whether physicians’ working with industry to develop new technology—and their participation in subsequent educational and promotional efforts—creates a conflict of interest that may cloud clinical decisions. These concerns are addressed, in part, by restrictions on the role of industry in the continuing education process and by the requirement that financial disclosures be made by physicians who provide educational services. The public disclosure of the extent of financial relationships between individual physicians and manufacturers through The Physician Payment Sunshine Act is intended to provide an added degree of accountability.
A more fundamental issue arises when physicians who provide clinical care also work with and are compensated by industry. Specifically, was the compensation received by the physician in exchange for legitimate services performed on behalf of a manufacturer, or was the payment made, in whole or part, to influence the physician to use or prescribe a particular item or service? In these cases, both the physician and the manufacturer risk potential allegations of violating the federal Anti-Kickback Statute (AKS), for which both the physician and manufacturer could face significant sanctions (to learn more about this statute, see “There Is No Such Thing as the Stark Anti-Kickback Statute”).
The purpose of this article is not to discourage physicians from contracting with manufactures to provide valuable services (eg, clinical trial investigator, advisory board member) that are crucial for the development of new technologies. That said, whenever there is a financial relationship between a physician and a manufacturer and the physician elects to prescribe or order the items from a manufacturer to treat patients, a question arises as to whether that financial relationship is appropriate. When a physician orders the item or service to treat a patient covered by Medicare, Medicaid, or another federal health care program, application of the AKS may become an issue.
THE THREE BASIC GUIDELINES
This is not a theoretical concern. There has been significant enforcement activity addressing this conduct during the past several years, with many manufacturers and physicians, including ophthalmologists, subject to harsh sanctions. There is, however, a positive message to go with this warning: By following three basic guidelines and learning from the cases that have been pursued in the past, both physicians and manufacturers should be able to minimize the risk of violation and resulting imposition of sanctions.
No. 1: The contract must be for legitimate, necessary services. A fundamental question in any kickback analysis is as follows: Was the payment for legitimate services that provided a real benefit to the manufacturer, or was the contract a sham that served only as a mechanism to pay the physician? The US government has frequently questioned contracts in which physicians were responsible for collecting and providing clinical data to manufacturers such as in a premarketing study, where the data were collected but never used. Similar questions have been raised when a manufacturer has contracted with dozens of physicians to provide consulting services but the need for only a few physician-consultants could be justified. Although the burden of ensuring the legitimacy of such contracts should rest with the manufacturer, a physician who is offered a consulting or data collection agreement would be wise to examine the arrangement closely to be comfortable that the agreement will generate legitimate, valuable services.
No. 2: The physician must perform the services and be able to document the performance. Having a written contract for the performance of a legitimate service for a manufacturer is not sufficient to justify payment. The physician must also perform the service contemplated under the contract. Performing the contracted services, however, may not be enough to avoid risk.
For many years, physicians have been counseled to maintain complete medical records for their patients, with the caution that, if it isn’t in the medical record, it didn’t happen (ie, the physician did not perform the service).
Physicians should follow the same advice when it comes to documenting the work performed under an agreement with a manufacturer. Some services generate their own documentation, such as performing a clinical trial that generates clinical records and data and a report of the findings. Other services, such as providing general consulting, may not. Physicians should therefore consider ways of documenting the performance of services so that they are prepared—if ever asked—to justify the payments received. A recent settlement involving a contract between a physician and an equipment distributor underscores this point (see Documenting Performance).
DOCUMENTING PERFORMANCE
An allegation was brought against an equipment distributor for paying physicians for services never performed. The distributor and the physicians challenged the allegation, but eventually the distributor and one of the physicians agreed to a multimillion-dollar settlement. In the final settlement agreement, the government described the conduct that it viewed to be problematic: “[Distributor] entered into consulting agreements with physicians … where services were not performed or not properly tracked, which resulted in remuneration in excess* of fair market value.”
In other words, despite a claim that the services were performed, the physician and distributor were held liable because neither could document those services. In the government’s view, it wasn’t in the record, so it didn’t happen.
*Emphasis added
No. 3: Payment for services must reflect their fair market value. Presuming that the services are reasonable and provide a benefit to the manufacturer, that they were performed, and that there is documentation to confirm that the services were performed, the final area of inquiry is whether the payment was reasonable for the services performed. Some contracts lend themselves to fixed payment amounts for discrete projects such as overseeing a clinical trial, drafting a scientific paper, or hosting an educational symposium. Many others lend themselves only to payment based on the time expended. Furthermore, even in the case of fixed payment agreements, the government often considers the amount of time expended by the physician to complete the project to determine if the payment was proper. As a result, the hourly rate has become a critical data point when analyzing the fair market value of a contract.
For many years, the physician community sought—without success—guidance on what hourly rate the government would consider to be reasonable for consulting and related services. Then, in 2007, a series of cases was settled with five major orthopedic medical device manufacturers that had been accused of paying kickbacks to physicians who ordered their devices. The government alleged that the physicians received unreasonably high payment amounts under consulting agreements, which constituted a violation of the AKS. After a lengthy investigation, the manufacturers agreed to settle, with payments for all five totaling several hundred million dollars. As part of the settlement, the manufacturers agreed to pay no more than a fair market rate for these consulting services. Fortunately, here the government articulated a standard to determine fair market value: Payments of up to $500/hour were acceptable, but companies were expected to make distinctions based on categories such as expertise and reputation. In other words, not every physician was expected to qualify for the $500 hourly rate. Additionally, the government acknowledged that, for some experts, even the $500 hourly rate might be inadequate. In these cases, the company could pay a higher hourly rate if it were supported by an independent valuation expert.
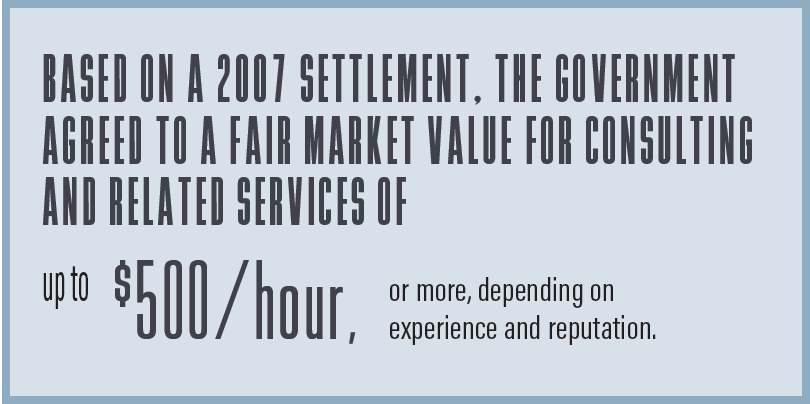
The resolution of that case has been used widely by manufacturers as the foundation on which to establish an hourly rate for physician consulting agreements. The $500 benchmark has been adjusted over time, and some manufacturers have sought assistance from valuation experts to establish a protocol to determine an appropriate rate for all physicians. Regardless of what methodology is used, physicians should take the initiative to be sure that the rate paid under any agreement can be justified as fair market value.
CONCLUSION
Contractual relationships between physicians and manufacturers are important to the development and assessment of new technology that benefits patients. Because these relationships generate payments to physicians who also make clinical decisions about which items or services to provide to their patients, the motivation for those clinical decisions can be questioned.
It is crucial for physicians to take steps to protect themselves as they provide these valuable services. Following the guidance presented in this article should help to give some degree of protection.




