
Premium IOL is an unofficial term that refers to IOLs that CMS has designated as presbyopia-correcting, astigmatism-correcting, or a combination of the two. IOLs that are not premium are generally referred to as conventional IOLs; these are primarily monofocal IOLs that provide distance vision but not near vision or astigmatism correction. Through Administrator Rulings CMS-05-01 and CMS-1536-R, CMS established that cataract surgery that is performed on a Medicare beneficiary and that includes the implantation of a premium IOL is a partially covered service under Medicare. This means that all items and services consistent with cataract surgery and the implantation of a conventional IOL are covered by Medicare and that the premium IOL and the services attributable to the premium IOL are not covered by Medicare and are therefore paid for by the patient.
CMS’ basis for designating premium IOLs and associated services as noncovered is the refractive benefit afforded by these lenses. Refractive surgery that serves to replace eyeglasses or contact lenses is not a benefit that is covered by Medicare, so the presbyopia-correcting and astigmatism-correcting functions of premium IOLs are deemed noncovered by CMS. Medicare beneficiaries may elect to pay out of pocket for the premium aspects of these IOLs. A list of premium IOLs is available at the CMS website and is updated regularly as the FDA approves new premium IOLs (for a current list, updated in June 2020, see Presbyopia-Correcting and Astigmatism-Correcting IOLs Recognized by CMS). CMS created the terms presbyopia-correcting IOL and astigmatism-correcting IOL for the payment policies described in the agency’s aforementioned rulings. These terms are not recognized by the FDA except for an acknowledgment of the CMS Premium IOL payment policy. This article explores covered and noncovered services with premium IOLs.
Presbyopia- and Astigmatism-Correcting IOLs Recognized by CMS
Presbyopia-Correcting IOLs
Alcon
- AcrySof IQ Restor (models SA25T0, SN6AD1, SN6AD3, MN6AD1, SV25T0)
- AcrySof IQ PanOptix Trifocal (model TFNT00)
- AcrySof IQ PanOptix UV Absorbing Trifocal (model TFAT00)
- AcrySof IQ Vivity Extended Vision IOL (model DFT015)
- AcrySof IQ Vivity Extended Vision UV Absorbing IOL (model DAT015)
Bausch + Lomb
- Crystalens
Johnson & Johnson Vision
- Tecnis Symfony (model ZXR00)
- Tecnis Symfony Plus OptiBlue (model ZHR00V)
- Tecnis Multifocal 1-Piece (models ZKB00, ZLB00, ZMB00)
- Tecnis Multifocal Acrylic (model ZMA00)
- Tecnis Multifocal Silicone (model ZM900)
- ReZoom (no longer available)
Astigmatism-Correcting IOLs
Alcon
- AcrySof IQ Toric (models SA6AT3, SA6AT4, SA6AT5, SN6AT3 through SN6AT9; collectively referred to as SN6ATT)
Bausch + Lomb
- Trulign Toric (models AT50T, BL1AT, BL1UT)
- enVista One-Piece Hydrophobic Acrylic IOL (models MX60T, MX60ET)
Johnson & Johnson Vision
- Tecnis Toric 1-Piece (models ZCT150, ZCT225, ZCT300, ZCT400, ZCT450, ZCT525, ZCT600)
- Tecnis Toric II 1-Piece (models ZCU150, ZCU225, ZCU300, ZCU375, ZCU450, ZCU525, ZCU600)
RxSight
- Light Adjustable Lens
STAAR Surgical
- Silicone 1-Piece Toric (models AA4203TF, AA4203TL)
Presbyopia- and Astigmatism-Correcting IOLs
Alcon
- AcrySof IQ Restor (models SND1T3, SND1T4, SND1T5, SND1T6, SV25T3-SV25T6)
- AcrySof IQ PanOptix Toric Trifocal (models TFNT30, TFNT40, TFNT50, TFNT60)
- AcrySof IQ PanOptix Toric UV Absorbing Trifocal (models TFAT30, TFAT40, TFAT50, TFAT60)
- AcrySof IQ Vivity Toric Extended Vision IOL (models DFT315, DFT415, DFT515)
- AcrySof IQ Vivity Toric Extended Vision UV Absorbing IOL (models DAT315, DAT415, DAT515)
Johnson & Johnson Vision
- Tecnis Symfony Toric (models ZXT150, ZXT225, ZXT300, ZXT375)
- Tecnis Symfony Plus OptiBlue Toric II (models ZHW150, ZHW225, ZHW300, ZHW375)
- Tecnis Multifocal Toric II (models ZKU150, ZKU225, ZKU300, ZKU375, ZLU150, ZLU225, ZLU300, ZLU375)
Source: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/PCIOL-ACIOL.pdf
EXPLANATION OF THE RULINGS
In its rulings, CMS discusses which services associated with premium IOLs are not covered and that are therefore billable to the patient. Both rulings (CMS-05-01, issued May 3, 2005, and CMS-1536-R, issued January 22, 2007) include similar statements.
In Ruling CMS-05-01, CMS states:
The presbyopia-correcting functionality of an IOL does not fall into the benefit category and is not covered. Any additional provider or physician services required to insert or monitor a patient receiving a presbyopia-correcting IOL are also not covered. For example, eye examinations performed to determine the refractive state of the eyes following insertion of a presbyopia-correcting IOL are noncovered.
These statements have been generally interpreted to mean that a premium IOL is not covered and that services required for the implantation and proper functioning of the premium IOL that are not performed as a part of conventional cataract surgery with an IOL are not covered. This applies to noncovered services provided by both the physician and the facility (ambulatory surgery center or hospital outpatient department). The rulings also state that these noncovered services are the patient’s financial responsibility. Because these services are not within a Medicare benefit category, physicians are not required to have patients sign an Advance Beneficiary Notice before furnishing a premium IOL for the patients to be financially responsible for the noncovered services. Nevertheless, from a risk management perspective, it is best to provide patients with clear notice of their financial obligation for the noncovered services.
SCOPE OF NONCOVERED SERVICES IN THE CONTEXT OF PREMIUM IOLS
Exactly what services associated with the implantation of a premium IOL are considered noncovered? Beyond the statements and example discussed earlier, CMS has not provided additional guidance on the subject except in the case of a femtosecond laser (discussed later). Nor has there been much scrutiny by CMS or others of precisely which noncovered services are attributed to the additional charges to the patient associated with premium IOLs by either the physician or facility. That said, the penalties for overcharging Medicare beneficiaries for covered services can be significant.
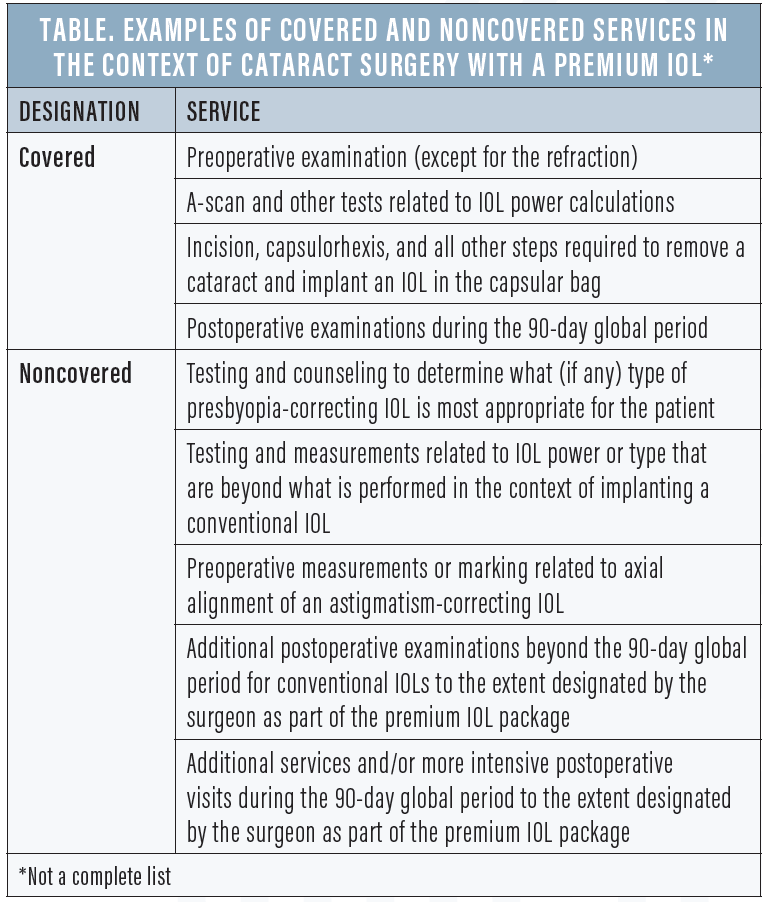
Generally speaking, Medicare covers tests, steps, and tasks that are a part of the protocol for cataract surgery with the implantation of a conventional IOL. These fully covered services extend to surgeries in which the implant is a premium IOL, and the standard Medicare payment for covered services plus the standard coinsurance are considered payment in full for this part of the overall service. Covered and noncovered services in premium IOL surgery are outlined in the Table.
The use of a femtosecond laser during premium IOL surgery can be considered noncovered only if it is performed on a limited and atypical basis during conventional IOL surgery. If a femtosecond laser is routinely used during both conventional and premium IOL surgery for steps such as the capsulorhexis and nuclear disassembly, then the laser’s use is considered covered.
CONCLUSION
CMS’ original ruling on presbyopia-correcting IOLs has been in place for 15 years without significant controversy. Physicians must understand that the core service is a Medicare-covered cataract procedure with implantation of an IOL, supplemented by a noncovered refractive benefit associated with the presbyopia-correcting or astigmatism-correcting aspects of the IOL and the overall service. Noncovered services that are being furnished to the patient and the associated charges for them should be clearly documented in the medical record and communicated to the patient before surgery.




