CASE PRESENTATION
A 63-year-old man was referred for cataract surgery on both eyes. The patient is an engineer by trade whose hobbies include race car driving and piloting a private aircraft. He reported a reduction in overall quality of vision and glare at night. The patient stated that he had enjoyed a visual acuity of 20/15 for most of his life and expressed frustration with the decline he had experienced during the past few years. He said that excellent distance vision was extremely important to him and that he did not want to wear reading glasses.
Several years earlier, the patient had undergone LASIK to correct myopia. During the current visit, he stated that it had taken three tries to “get it right” on the right side and only one try on the left. He said that he was not sure why multiple procedures were required for the right eye, and he had no record of the procedures or his previous refractions.
On examination, uncorrected distance visual acuity (UDVA) was 20/30 OU, which decreased to 20/80 OU with glare testing. A slit-lamp examination of each eye showed a normal anterior segment, a well-centered LASIK flap, and moderate nuclear sclerotic and cortical changes. The posterior examination was normal (Figures 1–3).
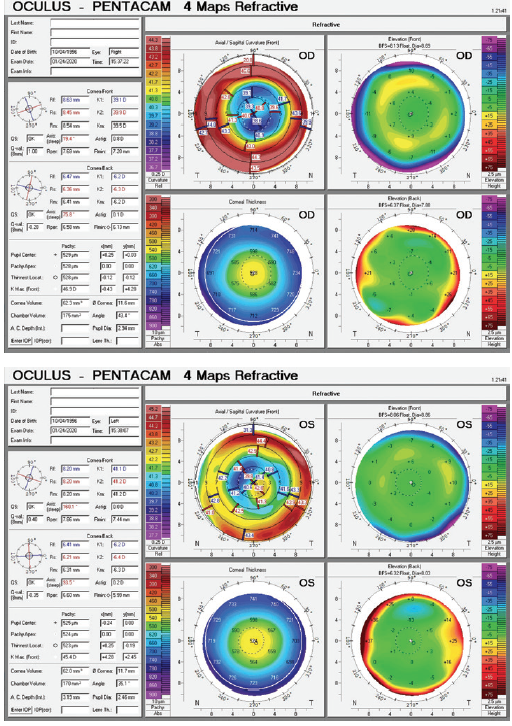
Figure 1. Imaging of both eyes with the Pentacam (Oculus Optikgeräte).
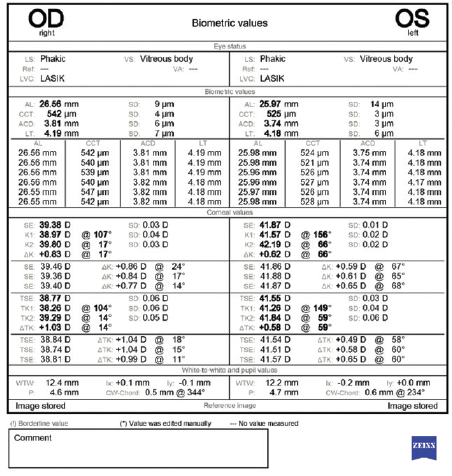
Figure 2. Biometry measurements performed with the IOLMaster (Carl Zeiss Meditec).
Figure 3. Macular OCT scans of both eyes.
After a discussion with the patient about the risks and benefits of cataract surgery and taking into consideration his desire for good vision at both distance and near, we decided to proceed with cataract surgery on the right eye and the implantation of a low-add multifocal IOL (AcrySof IQ Restor +2.5 D IOL, model SV25T0, Alcon). I used the ASCRS IOL power calculator for prior myopic LASIK/PRK to determine the IOL power (22.00 D) and confirmed the power with intraoperative aberrometry.
UDVA was 20/30 on postoperative day 1 and 20/50 at 1 week. Best corrected distance visual acuity was 20/20 with a manifest refraction of -1.50 D, and near visual acuity was J3. Two weeks later, the refraction was stable at -1.50 D, and an IOL exchange was performed without difficulty. The IOL power was calculated using the Barrett Rx formula, and a 20.50 D AcrySof IQ Restor +2.5 D IOL (model SV25T0) was placed. Initially UDVA was 20/25, but it was 20/40 at 1 month. Best corrected distance visual acuity was 20/20 with a manifest refraction of -1.00 D. Although the patient was quite frustrated with the outcome, we agreed to wait several weeks for the refraction to stabilize before deciding what action to take. During this time, a trifocal IOL was approved for use in the United States.
Two months after the initial IOL exchange, the patient returned to the OR for a second IOL exchange. I calculated the IOL power (20.00 D) using the Barrett Rx formula. The surgery was uncomplicated, and an AcrySof PanOptix IOL (model TNFT00, Alcon) was implanted. UDVA and uncorrected near visual acuity (UNVA) were 20/20 on postoperative day 1, and the patient was happy.
He is ready for surgery on the left eye but wants to undergo only a single procedure. How would you calculate the IOL power, and what type of IOL would you use?
—Case prepared by Brandon D. Ayres, MD

KENNETH A. BECKMAN, MD, FACS
IOL selection for patients who have a history of corneal refractive surgery is always a challenge. This patient underwent multiple refractive surgeries to attain satisfactory results, and two IOL exchanges in the right eye were required to reach his refractive goal. Obviously, the surgeon will want to minimize the chance that multiple surgeries on the second eye are required.
I generally avoid multifocal IOLs for post-LASIK patients for several reasons. First, as encountered in this case, it can be difficult to calculate IOL power accurately, and an error may necessitate additional surgery. Even the use of intraoperative aberrometry did not prevent a miscalculation here. Second, if the IOL power is correct, the patient may still experience significant postoperative aberrations, leading to dissatisfaction.
In this situation, I typically select a monofocal IOL. This patient and others like him are used to wearing readers because distance visual acuity was targeted at the time of their LASIK procedures. If a patient is comfortable with the idea of wearing readers, I target distance vision for both eyes. If a patient strongly wishes to minimize dependence on reading glasses, I consider monovision. I am comfortable explaining to patients that it may not be possible to achieve complete spectacle independence and that they may not be great candidates for some of the most recently available presbyopia-correcting lenses.
Because of the difficulty of obtaining accurate IOL power calculations in eyes with a history of refractive surgery, even with intraoperative aberrometry, it is worth considering a Light Adjustable Lens (RxSight) because refractive power can be adjusted postoperatively to address a miscalculation. Adjustments may be performed more than once, so the patient can test the result. This can be particularly useful for monovision because patients can titrate how myopic they want to be, depending on their needs.
There are several options for this patient. The easiest would be to place a traditional monofocal IOL. In my experience, patients similar to this one can do well with customized vision, including the implantation of a monofocal IOL in one eye and a multifocal IOL in the other. This is the approach I selected for my own cataract surgeries. If he chooses this approach but wants to ensure that no additional procedures are required, a Light Adjustable Lens and a distance target would be a great choice. I would prefer to avoid another multifocal IOL, even though this patient is satisfied with the multifocal lens in his right eye, because multiple surgeries were required to achieve that result.
He is a pilot, so crisp distance visual acuity is critical. He will probably see well at near with one multifocal IOL.
Had neither eye already undergone surgery, I would have recommended a Light Adjustable Lens for both. In my experience, this IOL seems to offer some subjective extended depth of focus. Distance vision could therefore be targeted for both eyes, or mini-monovision could be considered to provide good near vision without significantly compromising distance acuity.

H. BURKHARD DICK, MD, PHD, FEBOS-CR
This patient certainly has a problematic history. It seems to be crucial to achieve a successful outcome in the left eye with just one surgery. With an axial length of 26 mm, this eye was clearly moderately myopic before LASIK—probably 4.00 to 5.00 D.
Most striking of the left eye’s diagnostic parameters are the measurements with corneal topography in the area covered by the rather small pupil. Mainly due to a distinct inferotemporal steepening, the differences in height here are considerable: They will probably add up to about 4.00 D of astigmatism within this small central zone. I would obtain a corneal aberrometry measurement with the Pentacam HR for further quantitative evaluation of the abnormal corneal shape, with particular attention to coma and the total root mean square value. I would expect coma to be significant. I would also obtain measurements with the iTrace (Tracey Technologies) to distinguish corneal aberrations from lenticular aberrations.
Given that the left eye’s topography is more irregular by far than the right eye’s and that a difference in height of about 4.00 D can be expected within the pupillary diameter, implanting a multifocal IOL in an eye that has what amounts to a multifocal cornea (and certainly a high root mean square value) would be a poor choice. The risk that this patient, who has visually demanding hobbies, would be dissatisfied would be high. Removing an acrylic IOL for an exchange will probably require an incision that is larger than 3 mm, which may increase the amount of astigmatism and the magnitude of corneal aberrations in this eye. Implanting a toric IOL is not likely to correct the corneal irregularities fully.
Currently available IOL power calculation methods are inaccurate in many eyes that have undergone keratorefractive procedures. The literature on cataract surgery in this situation is limited and is usually based on small groups of patients. In their recent major review, Wang and Koch made clear that, despite the availability of new measurement technologies and the development of new IOL power calculation formulas, further advances are required to improve the outcome of cataract surgery on eyes that have a history of corneal refractive surgery.1
I would inform this patient about an alternative to multifocal IOLs, one I believe would give him a decent chance of postoperative satisfaction: the small-aperture IC-8 (AcuFocus, not available in the United States). This one-piece hydrophobic acrylic IOL is intended for implantation in the capsular bag. The lens features an embedded annular mask that has an outer diameter of 3.23 mm and a central aperture measuring 1.36 mm in diameter. This IOL recently became commercially available in low powers. I believe it would be an appropriate choice, given the aberrations in the central cornea. The left eye seems to be the patient’s nondominant eye, which is usually the one that receives the small-aperture lens. UDVA with an IC-8 is excellent. In a comparative clinical trial including 76 eyes, UDVA with this technology was superior to that achieved with a different type of extended depth of focus IOL (Tecnis Symfony, Johnson & Johnson Vision).2 This patient’s chances of spectacle independence are therefore good.
The optical properties of the IC-8 and their potential implications for daily life—specifically for aviation and race car driving—must be discussed in detail with the patient before surgery. A postoperative reduction of contrast sensitivity is likely to occur. I have found that the hypothetical loss of brightness due to the annular mask, on the other hand, is often less than expected after the implantation of a small-aperture device, probably due to neural adaptation.
It would be important to check, however, whether wearing a small-aperture IOL is compatible with the Federal Aviation Administration’s requirements for the renewal of a pilot license; this is probably the aspect of surgery most dear to the patient’s heart.

SUMITRA S. KHANDELWAL, MD
Credit is due Dr. Ayres for taking such a detailed approach to this patient whose visual demands are high. My first reaction is to take a step back in regard to his history of multiple refractive surgeries in an attempt to “get it right.” Was this an issue of consecutive hyperopia, residual myopia, or chasing his expectations? The central cornea of the right eye looks very flat, and this may not be a patient in whom I would have implanted a multifocal IOL. It is wonderful that UDVA and UNVA were 20/20 and the patient was happy 1 day after the second IOL exchange in the first eye, but this patient’s history has shown that his visual acuity on day 1 often is not the same a few weeks later. My first question would be what the visual acuity and refraction were a few weeks later.
For the second eye, I would counsel the patient that no one can guarantee a single procedure for him based on how cataract surgery went on his first eye and his history of multiple refractive surgeries. The cornea of the left eye presents a challenge. It looks highly aberrated with the central 3 mm on topography spanning 3.00 D of astigmatism. I would show him the topographic maps of his two eyes side by side and give him a choice: match their results by implanting an AcrySof PanOptix IOL in the left eye (with the risk of an IOL exchange if visual acuity is not clear), or implant a monofocal IOL with a target of distance vision. That said, if he is currently happy with the UDVA, UNVA, and uncorrected intermediate visual acuity in the right eye, I would recommend implanting an AcrySof PanOptix IOL in the left eye.
Although I generally base IOL selection for the second eye on outcomes with the first eye, this situation is different because the corneas of the left and right eyes are so different. I would use the ASCRS IOL power calculator for prior myopic LASIK/PRK and focus on results with the Barrett True-K formula. I would also counsel the patient preoperatively about his possible future need for an IOL exchange.


RAPHAEL PENATTI, MD, AND GEORGE O. WARING IV, MD, FACS
The sagittal curvature map for the left eye shows an irregular cornea with significant higher-order aberrations, specifically positive spherical aberration and coma. For eyes with irregular corneas and large amounts of coma, we generally prefer to implant an aberration-free IOL. In other words, instead of trying to neutralize the spherical aberration found in this cornea, we would try not to add aberrations to the optical system. It is possible that these corneal irregularities give this patient functional near vision through pseudoaccomodation. We would take advantage of the UNVA in the postsurgical eye and would counsel him that he may have an increased need for reading glasses after cataract surgery on the left eye.
Like Dr. Ayres, we rely on the ASCRS IOL power calculator for prior myopic LASIK/PRK in situations similar to this one. We would pay close attention to the results with the Barrett True-K No-History formula, and we would perform intraoperative aberrometry.
Given the past difficulties achieving the intended refractive targets for this patient, we would thoroughly counsel him on the possible future need for an IOL exchange or laser vision correction (PRK with mitomycin C applied for an extended duration) to address a residual refractive error and would document this discussion in the medical record.

WHAT I DID: BRANDON D. AYRES, MD
Prior to surgery, the patient and I had a long discussion emphasizing that, even with the best calculations, there was a chance that he would need an IOL exchange. The Barrett True-K formula and the ASCRS IOL power calculator for prior myopic LASIK/PRK agreed that the implantation of a 17.50 D AcrySof IQ PanOptix trifocal IOL (model TNFT00) should produce an outcome near emmetropia. Intraoperative aberrometry was used to confirm the IOL power during surgery.
One day after surgery, distance and near visual acuity was 20/20 with a plano refraction. Refractive stability has been maintained. The patient has been happy, but he recently noticed that the visual acuity in his left eye is slightly better than in his right (20/20-1). He wants to know what can be done to further improve visual acuity in his right eye.
1. Wang L, Koch DD. Intraocular lens power calculations in eyes with previous corneal refractive surgery: review and expert opinion. Ophthalmology. Published online June 29, 2020. doi:10.1016/j.ophtha.2020.06.054
2. Schojai M, Schultz T, Jerke C, Böcker J, Dick HB. Visual performance comparison of 2 extended depth-of-focus intraocular lenses. J Cataract Refract Surg. 2020;46(3):388-393.




