There is not a single best universal method for cataract surgery. There is, however, the best method for an individual surgeon. My preferred routine cataract surgery technique, shown in an accompanying Eyetube video (bit.ly/mccabe0220), starts with isolating the lid margin and lashes away from the sterile field (Figure 1). I consider this to be the most important step in the early stage of surgery.
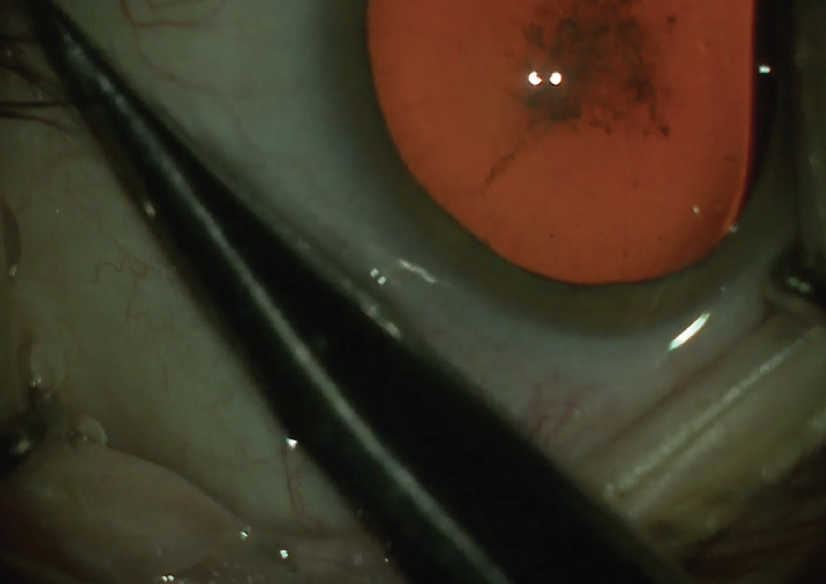
Figure 1. The lashes are isolated from the surgical field.
INCISIONS AND CAPSULORHEXIS
Once I achieve a sterile surgical field, I use a 1.2-mm stiletto diamond blade (Mastel) to enter the anterior chamber, which I fill first with preservative-free lidocaine or lidocaine with phenylephrine and then with a dispersive OVD. I like to use DuoVisc (Alcon), so at this stage I use the Viscoat component of that dual-OVD product.
I then use 0.12-mm forceps to stabilize the eye while I create a stab incision parallel to the iris with a 2.2-mm diamond blade (Mastel). To create the capsulorhexis, I use Utrata forceps, which have a sharp tip. I use that tip to score and then grab the anterior capsule. Usually with about three grasps of the capsule I can create a 5.5-mm circular, well-centered capsulorhexis (Figure 2).
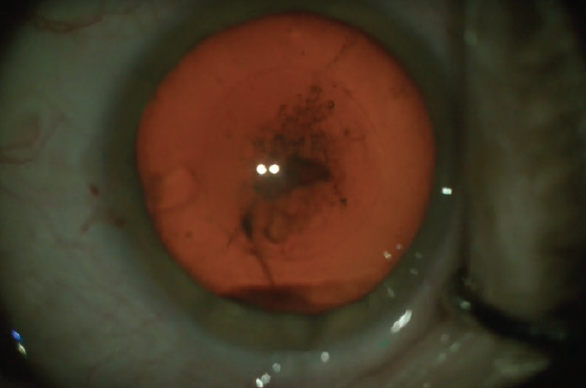
Figure 2. A 5.5-mm circular and central capsulorhexis.
I use a Chang hydrodissection cannula (Katena) to separate cortex and the nucleus from the capsule, after which I like to confirm a little rotation before ending that part of the procedure.
PHACOEMULSIFICATION
During nucleus division and phacoemuslfication I make sure that the ports on the infusion sleeve point horizontally, rather than toward the corneal endothelial cells or the posterior capsule. I prefer to use a vertical chop technique. I place a 45º Kelman 0.9-mm phaco tip through the main incision and, with the phaco tip in my right hand and a Nichamin chopper in my left through the paracentesis, I engage the nucleus to create segments. I then grasp the segments and emulsify each piece individually with slightly higher vacuum and torsional phaco power.
Sometimes I use a horizontal chopping motion, which I find can be helpful to free and emulsify the nuclear segments. With a little bit of vacuum, I then remove the epinucleus.
While the scrub technician changes the infusion to the 0.3-mm polymer I/A tip from Alcon, I take a 27- or 30-gauge cannula filled with balanced saline solution and use it to irrigate and loosen the cortex (Figure 3). This gives me something to do while the OR staff prepares the I/A handpiece, and I find that this step eases removal of the cortical material. I remove cortical material using a shearing or tangential motion.
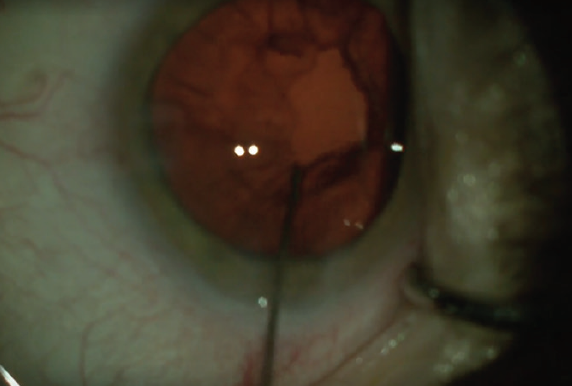
Figure 3. A cannula filled with balanced saline solution is used to irrigate and loosen the cortex.
The cannula is then refilled with balanced saline solution, and I use it to do what I call power washing of the posterior capsule to remove any remaining lens epithelial cells. Like any maneuver completed with a cannula, it is important to hold onto the hub of the cannula to ensure that it does not become a projectile.
After I fill the capsular bag with ProVisc, which is the cohesive portion of the DuoVisc OVD, I like to use a double-sided polisher (Epsilon Instruments) because I can flip it over to polish the lens epithelial cells from the posterior capsule and the anterior capsular leaflet. I place the lens inserter for the lens of choice temporally into the wound. In the case presented in the video, I implanted an Akreos IOL (Bausch + Lomb).
I use a wound-assisted technique, and I try not to stretch the wound. This is easily accomplished with the Akreos, which fits nicely through a 2.2-mm incision. I then remove any OVD from in front of and behind the lens using the I/A tip. In the posterior plane, I make sure to point the port up toward the lens so that it doesn't engage the posterior capsule. The exception is when I am trying to remove any little bits of residual cortex that I see. In the video, it is clear to see that I made a nice central 5.5-mm capsulorhexis because it barely overlaps the optic.
At the end of the case, I hydrate the roof and floor of the incisions and check the IOP by making a small indentation with the cannula; I try to keep the pressure in the low 20s.
I also add 0.10 mL intracameral moxifloxacin (Vigamox, Alcon) prior to the final IOP check, and I might release a little pressure just to get it exactly perfect. Lastly, I check the integrity of the incisions to make sure they are not leaking and add a topical drop of moxifloxacin before ending the surgery (Figure 4).
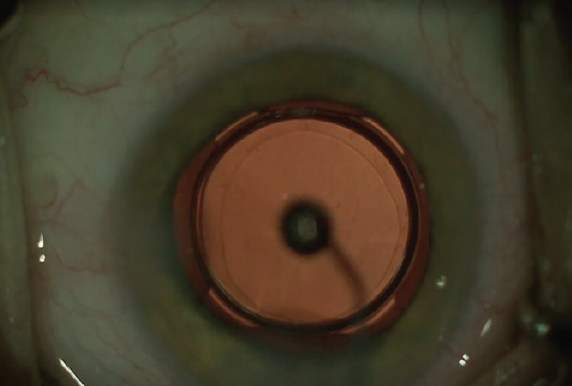
Figure 4. Moxifloxacin is applied topically.
I apply a little bit of the residual moxifloxacin topically onto the eye, and I add an IOP-lowering drop of Combigan (brimonidine tartrate 0.2%/timolol maleate ophthalmic solution 0.5%, Allergan) as well.
CONCLUSION
After years of performing cataract surgery, I have developed the routine technique described here, which for me is safe, effective, and efficient. The exact procedure I use will undoubtedly be different from case to case, but I stick with my go-to methods as much as possible while maintaining the safest surgery possible.