Modern cataract extraction is generally a safe surgical procedure, with few patients experiencing adverse events or complications. Some cataract cases, however, still require careful consideration and planning due to the nature of either the patient or the cataract or both. These cases frequently consume more operative time, require specialized instrumentation or depth of surgical principles, and come with a higher risk of complications. This article describes three challenging types of cataract presentations and some of the surgical techniques that can be used to approach them.
THE WHITE CATARACT
Explanation. Multiple etiologies can create a white cataract (Figure 1), when the lens takes on a formidable milky white appearance. Because of their opaque nature, these cataracts generally tend to cause significant visual disability for the patient, and they can pose serious challenges for the novice (and even advanced) surgeon attempting to remove them.
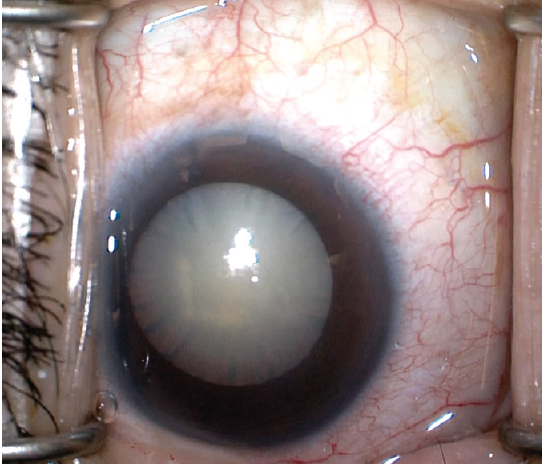
Figure 1. A white cataract has a formidable milky white appearance.
Potential problems. The first, and likely most obvious, problem is that the retinal red reflex is severely blunted, if present at all. This makes visualization for performing the continuous curvilinear capsulorhexis nearly impossible. Use of trypan blue dye to stain the anterior capsule is strongly recommended to provide the view necessary for successful capsulorhexis completion.
The potential in these eyes for raised intralenticular pressure may be recognized during the preoperative examination if the anterior capsule is bulging anteriorly. This finding is particularly dangerous because creating a break in a pressurized capsule may result in a radial capsular tear, producing the so-called Argentinian flag sign, in which the white cataract is in the center of the tear with the stained blue capsule on either side (Figure 2). The tear can then travel through the equator around to the posterior capsule, leading to serious complications such as zonular rupture, posterior capsular rupture, vitreous prolapse, and dropped nucleus.
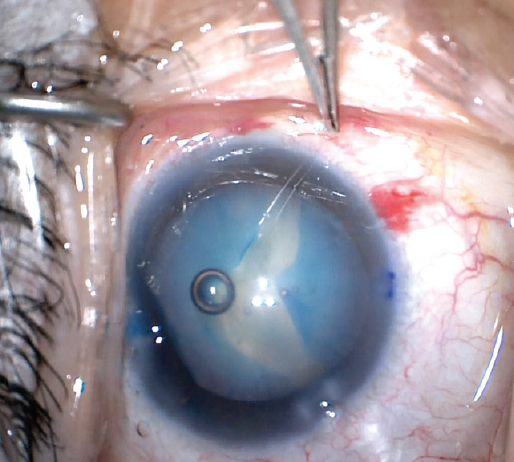
Figure 2. The Argentinian flag sign highlights the white cataract against the blue-stained capsule.
Recommended surgical techniques. Several techniques can be used intraoperatively to minimize the risk for radial extension of the capsular incision, and two are described here.
In the first, to flatten the capsule, the eye should be properly pressurized with a cohesive OVD, ideally a high-viscosity OVD such as Healon GV or Healon 5 (both by Johnson & Johnson Vision). These high-molecular-weight OVDs apply pressure on the capsule and minimize the pressure differential between the inside and outside of the capsular bag.
In the second, needle decompression is performed to depressurize the capsule. In this technique, the capsule is punctured with a 27- or 30-gauge needle while the plunger is simultaneously withdrawn to aspirate the liquified cortex, thereby lowering intracapsular pressure and theoretically lowering the risk of a radial tear (Figure 3).1
Both techniques aim to equalize the pressure inside and outside of the capsular bag to dissipate the driving force for radial extension of the capsulorhexis. They can be used independently or in combination.
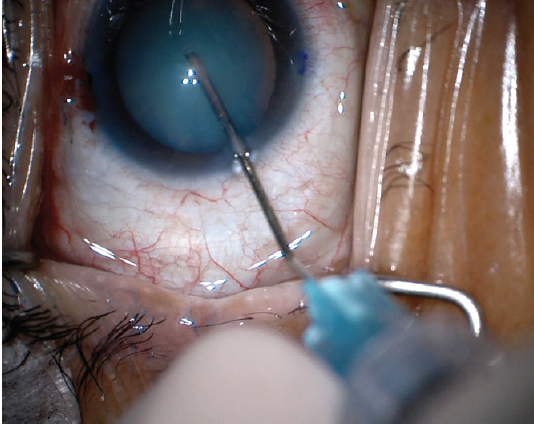
Figure 3. Needle decompression can be used to depressurize the capsule and theoretically lower the risk of a radial tear.
Capsulorhexis. Generally the capsulorhexis should be started smaller than in normal cases because it will tend to increase in diameter and may end up under the iris if started too large. Alternatively, a femtosecond laser can be used to assist in creating the capsulorhexis.
A two-step technique involving the creation of a laser mini-capsulorhexis with a 2-mm diameter to release the intralenticular pressure prior to wound construction has also been described.2 Neither this two-step femtosecond laser technique nor the standard laser-assisted capsulorhexis have been studied extensively in intumescent white cataracts, but there is some initial evidence of increased safety.2,3
Phacoemulsification. The nucleus of the white cataract can be hard or soft. In the case of a very soft nucleus, the capsular bag can be extremely floppy and present itself to the phaco tip even with low vacuum settings. To minimize this risk, we recommend using a supracapsular technique, in which the nucleus is prolapsed into the anterior chamber before emulsification and aspiration.
A very hard nucleus, on the other hand, may require setting the phaco machine to a dense lens setting, employing chop techniques, and manually disassembling the nucleus into more pieces than usual to allow safe emulsification. Modulation of ultrasound energy with pulse or burst phaco mode can increase the efficiency of the ultrasound,4 but more total phaco energy than usual may still be needed for the removal of a hard nucleus.
It is important to remember that, with increased phaco energy, also called cumulative dissipated energy, comes an increased risk of phaco burn at the level of the cornea.4 Similarly, if the nucleus pieces are sharp, it is important to guard against their tumbling in the capsular bag and to bring the pieces into the anterior chamber if warranted.
Other considerations. In the event that the surgeon anticipates a very hard nucleus, he or she can use an OVD with dispersive properties to protect the endothelium. One disadvantage with this type of OVD is that it is harder to remove from the eye at the end of the case, and postoperative IOP spikes can result if some is left behind.
Finally, if the lens is very dense, one may consider using or converting to an extracapsular cataract extraction or small-incision cataract surgery technique. By taking one’s time, applying the above techniques, and being prepared, the surgeon will be in the best possible position to tackle the challenge of the white cataract.
THE POSTERIOR POLAR CATARACT
Explanation. Another challenging presentation with a heightened risk for intraoperative complications is the posterior polar cataract. This type of cataract, located in the subcapsular region of the lens, may often be adherent to a thin posterior capsule (Figure 4). Adherence to the posterior capsule can be assessed preoperatively with imaging studies such as anterior segment OCT, ultrasound, or Scheimpflug imaging. These types of cataracts often form early in life, but, because of the location of the opacities, they tend to become more visually disabling and clinically significant over time.
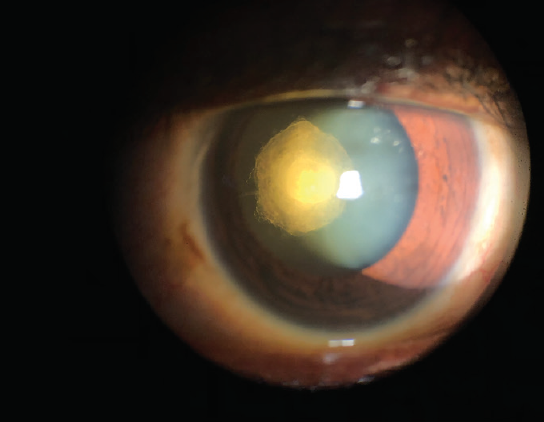
Figure 4. A posterior polar cataract, located in the subcapsular region of the lens, is often adherent to a thin posterior capsule.
Potential problems. The increased risk in removing a posterior polar cataract comes from the potential for tearing the posterior capsule while attempting to dissect or delineate planes, which can lead to vitreous prolapse and lens drop.5 The patient should be counseled at the preoperative evaluation about the higher potential for complications and the likely need for a postoperative Nd:YAG laser posterior capsulotomy to remove residual plaque.
Recommended surgical technique. No surgical technique will eliminate the possibility of posterior capsular rupture, but the key to minimizing this risk is to perform hydrodelineation instead of hydrodissection. Hydrodissection with a posterior polar cataract has the potential to blow out the back of the capsule, instead of the intended effect of separating the cortex from the nucleus, because the fluid wave will be directed posteriorly by the adherent lens fibers. Instead, hydrodelineation is performed to separate the endonucleus from the epinucleus.
Inside-out hydrodelineation can separate the core of the nucleus without the risk of inadvertent fluid injection into the subcapsular plane.6 In this maneuver, a central trench is sculpted in the vertical plane, then fluid is rapidly injected with a right-angle cannula into the right wall of the trench. One of the benefits of this technique is that the surgeon can achieve a desired amount of epinuclear cushion by choosing the level of fluid injection. Fluid travels from the inside out, and a successful delineation is marked by a golden ring within the lens (Figure 5).
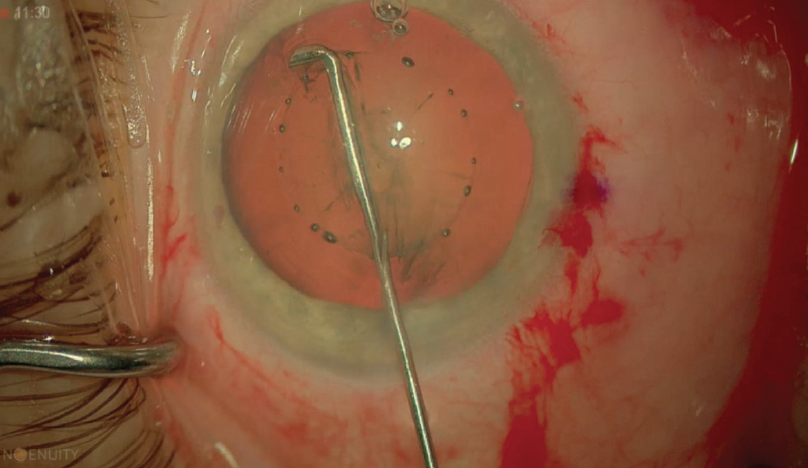
Figure 5. Successful hydrodelineation is indicated by a golden ring within the lens.
Capsulorhexis. The capsulorhexis in these eyes should be around 5 mm. A smaller opening may make prolapse of a soft nucleus difficult, and it comes with a higher risk of capsular phimosis. A larger opening may not provide the necessary support for a sulcus-situated IOL in the event of posterior capsular rupture.5
Phacoemulsification. During nucleus removal, rotation of the lens material should be kept to a minimum. Low I/A settings are recommended to minimize turbulence. After removal of the endonucleus, the lens material is aspirated from anterior to posterior, starting with the epinucleus and leaving the central posterior cortex for last. Again, a low aspiration flow rate is recommended. Multifocal hydrodissection or viscodissection with a dispersive OVD can be used to elevate the epinucleus before aspiration. Because the capsule is no longer full, the fluid wave does not pose a threat to the weakened posterior capsule.5
Polishing of the posterior capsule in these eyes is not recommended due to its fragility. If the patient’s vision is affected, Nd:YAG laser posterior capsulotomy can be performed at a later time when the eye is a closed system.
Other considerations. Provided the capsule is still intact, the case can be completed with injection of a one-piece acrylic IOL. If the capsule is ruptured during the case, the surgeon should have a dispersive OVD ready to tamponade the break and prevent vitreous prolapse. Limited anterior vitrectomy should then be performed. If the posterior capsule is ruptured but the capsulorhexis will provide adequate support, a three-piece IOL placed in the ciliary sulcus is the best option. If that is not possible, an anterior chamber IOL or a sutured IOL can be used.5
CATARACT IN PATIENTS WITH UVEITIS
Explanation. Patients with uveitis develop cataracts earlier than other patients due to inflammation and corticosteroid use.
Potential problems. Inflammatory changes in these eyes make surgery more technically challenging and more prone to postoperative complications such as cystoid macular edema, posterior capsular opacification, recurrent inflammation, and epiretinal membrane development.7 However, good visual outcomes can still be achieved with the use of careful preoperative management, surgical technique, and postoperative care.
Recommended surgical techniques. The most important step in achieving a good outcome is controlling preoperative inflammation (Figure 6). There is consensus that the eye should be inflammation-free for 3 months before surgery.7-9 This is generally achieved with the use of topical or systemic corticosteroids, but the side effects of long-term systemic corticosteroids can be severe. To reduce side effects while still controlling inflammation, the use of NSAIDs may be helpful.8,9
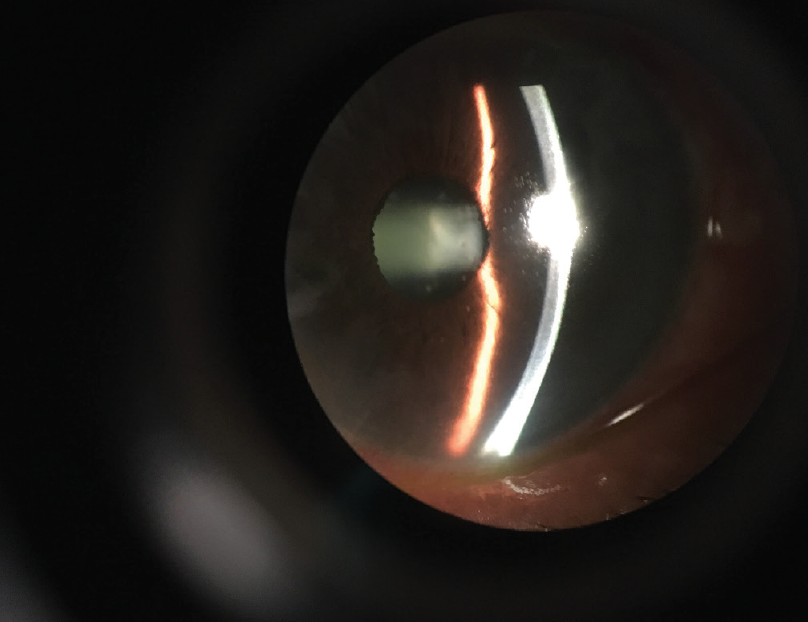
Figure 6. The most important step in achieving a good outcome in a patient with uveitis is controlling preoperative inflammation.
Capsulorhexis. Intraoperatively, posterior synechiae may be encountered in many patients with uveitis. These adhesions between iris and anterior capsule will limit pupil dilation and prevent the surgeon from accessing the anterior capsule to perform capsulorhexis. Before the capsulorhexis is undertaken, synechiolysis can be performed, mechanically breaking the adhesions with a blunt object such as an OVD cannula or a spatula. Note that these adhesions can be present peripherally, beyond the pupillary margin, so the instrument must be inserted deep enough to ensure full separation of the iris from the anterior capsule. Any membrane present on the pupil can be gently removed using microforceps.
After the synechiae are broken, the iris still may not dilate sufficiently if only topical mydriatics are used. Intracameral mydriatics, such as the combination of 1% phenylephrine and 0.3% ketorolac (Omidria, Omeros) can be useful in these cases. This formulation, mixed into the irrigation solution, has been shown to reduce postoperative pain, prevent intraoperative miosis, and decrease perioperative complications.10-12
If the iris does not respond to pharmacologic agents, mechanical dilation may be necessary using iris hooks or a pupil expansion ring such as the Malyugin Ring (MicroSurgical Technology). Intracameral lidocaine or additional intravenous sedation may make patients feel more comfortable during mechanical dilation, as otherwise they may be able to feel the pupil stretch.
In these instances, the Malyugin Ring has advantages over iris hooks. First, iris hooks require four additional paracentesis wounds, whereas the ring can be placed through the primary incision. Second, the ring will keep the pupil round during the case, whereas the four iris hooks will pull the pupil into a square, potentially traumatizing the iris. Some surgeons prefer to use five iris hooks because it causes less iris tenting and a more rounded pupil; however, it requires more time and more corneal incisions. Third, placing iris hooks in a square configuration tents the iris above the main incision into the path of the phaco tip, making the case more challenging during phacoemulsification and complicating aftercare.
Patients with uveitis may also require trypan blue staining of the capsule before creation of the capsulorhexis. Note that the trypan blue must be added after the pupil is dilated.
Phacoemulsification. This is preferred over extracapsular cataract extraction in individuals with uveitis because of the lower incidence of postoperative inflammatory complications, presumably because phacoemulsification requires less iris and wound manipulation.8,9
Other considerations. Another change that can occur in patients with chronic inflammation is weakening of the zonular fibers that suspend the lens. Signs of zonular laxity include easy lens movement (phacodonesis), the formation of capsular ripples in front of the leading edge of the capsulorhexis, a previously round capsulorhexis becoming oval, and trampolining of the posterior capsule during nucleus and cortex removal.
Patients with weak zonules are at increased risk for lens subluxation, capsular tears, and vitreous loss, but these risks can be mitigated with the use of capsular support devices. Capsular hooks can be placed through the capsulorhexis to stabilize the capsular bag during phacoemulsification, and a capsular tension ring (CTR) can be placed into the capsular bag after cortex aspiration to provide permanent support. The CTR in the bag will push the capsule equator outward, distributing tension equally over the intact zonules.13 In addition to lowering the risk of lens subluxation, the CTR may also decrease the incidence of capsular phimosis and posterior capsular opacification and facilitate Nd:YAG capsulotomy.14 These capsular support devices may not be required for all patients with uveitis, but the surgeon should be prepared to use them if necessary.
CONCLUSION
The goal of this article is to educate budding cataract surgeons about the importance of pre- and postsurgical preparation. Pre- and postoperative debriefing can pay dividends intraoperatively for the patient currently in front of you and for the thousands of patients to come. With the correct tools, mindset, and approach, any cataract can be conquered successfully.
The steps outlined above represent a departure from normal now; however, the types of difficult cataracts described here, and others, will not be viewed as difficult in the future but rather as challenging, testing your presurgical and intraoperative decision-making capacities.
1. Nabil KM. Lens decompression technique for prevention of intraoperative complications during phacoemulsification of intumescent cataract. Indian J Ophthalmol. 2017;65(12):1436-1439.
2. Schultz T, Dick HB. Laser-assisted mini-capsulotomy: A new technique for intumescent white cataracts. J Refract Surg. 2014;31(11):742-745.
3. Conrad-Hengerer I, Hengerer FH, Joachim SC, Schultz T, Dick HB. Femtosecond laser-assisted cataract surgery in intumescent white cataracts. J Cataract Refract Surg. 2014;40(1):44-50.
4. Devgan U. Surgical techniques in phacoemulsification. Curr Opin Ophthalmol. 2007;18(1):19-22.
5. Vasavada AR, Vasavada VA. Managing the posterior polar cataract: An update. Indian J Ophthalmol. 2017;65(12):1350-1358.
6. Vasavada AR, Raj SM. Inside-out delineation. J Cataract Refract Surg. 2004;30(6):1167-1169.
7. Llop SM, Papaliodis GN. Cataract surgery complications in uveitis patients: a review article. Semin Ophthalmol. 2018;33(1):64-69.
8. Van Gelder R, Leveque TK. Cataract surgery in the setting of uveitis. Curr Opin Ophthalmol. 2009;20(1):42-45.
9. Baheti U, Siddique SS, Foster CS. Cataract surgery in patients with history of uveitis. Saudi J Ophthalmol. 2012;26(1):55-60.
10. Hovanesian JA, Sheppard JD, Trattler WB, et al. Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain: Integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg. 2015;41(10):2060-2068.
11. Donnenfeld ED, Whitaker JS, Jackson MA, Wittpenn J. Intracameral ketorolac and phenylephrine effect on intraoperative pupil diameter and postoperative pain in cataract surgery. J Cataract Refract Surg. 2017;43(5):597-605.
12. Rosenberg ED, Nattis, AS, Alevi D, et al. Visual outcomes, efficacy, and surgical complications associated with intracameral phenylephrine 1.0%/ketorolac 0.3% administered during cataract surgery. Clin Ophthalmol. 2018;12:21-28.
13. Hasanee K, Butler M, Ahmed IIK. Capsular tension rings and related devices: Current concepts. Curr Opin Ophthalmol. 2006;17(1):31-41.
14. D’Eliseo D, Pastena B, Longanesi L, Grisanti F, Negrini V. Prevention of posterior capsule opacification using capsular tension ring for zonular defects in cataract surgery. Eur J Ophthalmol. 2003;13(2):151-154.