I was recently invited to join a Booth Talk during the American Academy of Ophthalmology’s annual conference to discuss the current practice of IOL calculations and to present a patient case study demonstrating my experience with the IOLMaster 700 (ZEISS). In my practice, and in discussions with patients and colleagues, IOL calculations have been a major topic during the planning phases of cataract surgery—and rightfully so, as this process has become one of the most critical stages in determining treatment success rates. In the past decade, studies have demonstrated several causes of inaccuracies in lens calculations.1 Sources of error include effective lens positioning (ELP 35%), axial length (27%), and refraction (17%).2 During the panel, Douglas Koch, MD, raised an interesting question: “How accurately are we really calculating IOLs for our cataract patients?” He further claimed that, currently within clinical practices, we are achieving approximately 70% accuracy within a half diopter. According to Dr. Koch, our calculations are much worse when facing more complex cases. These leave us with the knowledge that there are definite opportunities for significant improvement.
Measuring the Cornea
Compared to previous methods of determining lens accuracy, new factors have emerged to determine stronger IOL calculations. ELP is still a major focus, but cornea—both anterior and posterior—has now moved to the forefront. Refraction still remains a challenging area, but we no longer consider axial length measurements as an issue thanks to swept-source OCT (SS-OCT) devices that offer superior accuracy of optical biometry. Still, how can we improve lens power calculations?
Traditionally, we calculate lens power by using variants of the vergence formula identifying the variable factors or by using a combination of vergence formulas including the Barrett Suite, Holladay 1 and 2, the Hoffer Q, SRK/T, Haigis, and others such as ray tracing and AI. What are all of these really trying to do? More or less, they are still trying to figure out the ELP; the challenge is that accurate calculations still elude us. Physicians have looked at OCT for measuring and estimating ELP, but none of the studies demonstrated an improvement in accurate ELP calculation.
What is the best practice today? In my practice, the emerging importance of factoring all possible measurements, including the cornea, has shaped the current paradigm, allowing more accurate IOL predictions. You want to measure the cornea using multi-zone LEDs. This provides not only quantitative information but also qualitative information about the posterior corneal curvature, the anterior cornea, and corneal thickness. So, we use multi-zone LEDs, and we aim to measure the posterior cornea and combine it with the best formulas, such as the Barrett Suite and Holladay Consultant. By using this process, we see a big improvement with normalized and abnormal corneas, which helps with target calculations. A major shift is evident in the IOLMaster 700 (ZEISS), which allows total keratometry (TK) using two exclusive new formulas: Barrett TK Universal II and Barrett TK Toric. The technology can also be used with existing IOL calculation formulas and has shown favorable results in increasing accuracy in refractive outcomes.3
Patient Case Study
A 74-year-old woman was referred to me for cataract surgery. In addition to a visually significant cataract, she presented with high myopia and was diagnosed with mild myopic macular changes by our in-practice retina specialist. I conducted a full dry eye workup including tear osmolarity and MMP-9 testing in both eyes. Her tear osmolarity and her MMP-9 were abnormal. This indicated inflammation on the surface along with moderate meibomian gland dropout discovered on her meibography. These results led me to educate the patient on her coexisting diseases: dry eye disease (DED) and cataract.
After addressing the patient’s DED, I brought her back in for her biometry, topography, and keratometry. For her cataract, she chose an extended depth of focus (EDOF) lens, understanding that she may still need mild glasses for reading and a potential glare/halo issue. When calculating IOL, I look at a lot of data and spend time calculating each one of my patients’ IOLs personally. With this patient, I looked at her Delta K and it was 0.53 D. Typically, I treat cylinder between 0.50 D and 1.0 D with a limbal relaxing incision (LRI) and anything over 1.0 D with a toric IOL if the astigmatism has a symmetric pattern. I looked at TK and it was 0.39 D, leading me away from performing an LRI. The power we selected using the Barrett Universal TK formula was +5.0 D for a refractive target of -0.27 D (Figure 1).
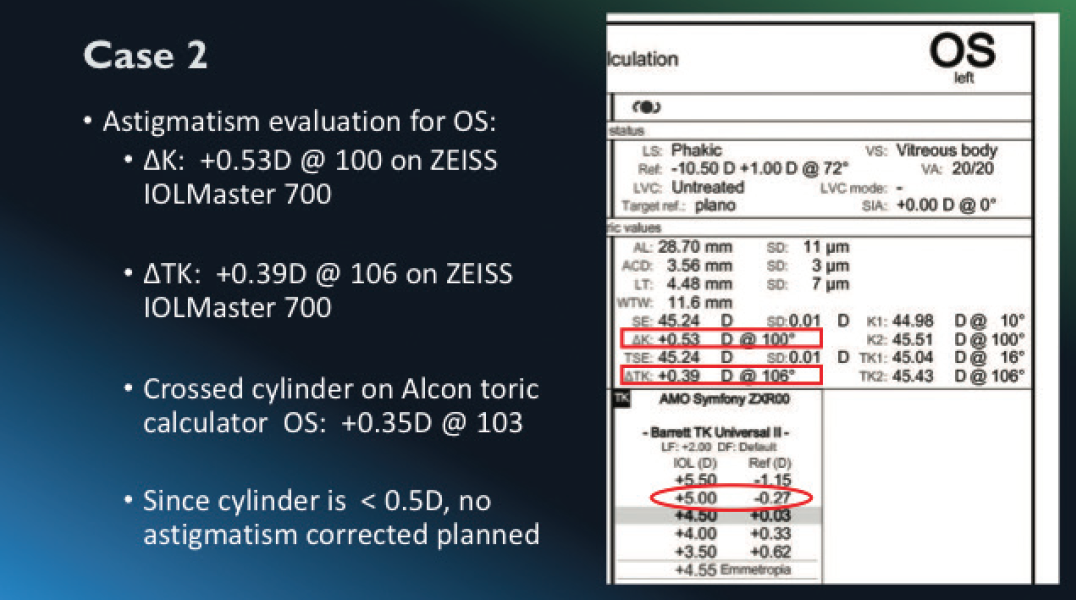
Figure 1. The power we selected for this patient using the Barrett Universal TK formula was +5.0 D for a refractive target of -0.27 D.
IOL Centration: Measuring Angle Kappa and Angle Alpha
One of the interesting aspects of this case was the patient had a significant angle kappa—the distance between the pupil center and the visual axis. This is particularly important because the center of the pupil is no longer the point where fovea-centric light passes (Figure 2). One of the benefits of the IOLMaster 700 is the consideration of angle kappa, which is represented as a CW chord. This patient’s P-distance measurement was 0.45 mm, making her a good candidate for a diffractive lens. Having this type of information helps determine the best possible calculation and customization for IOL implant selection.
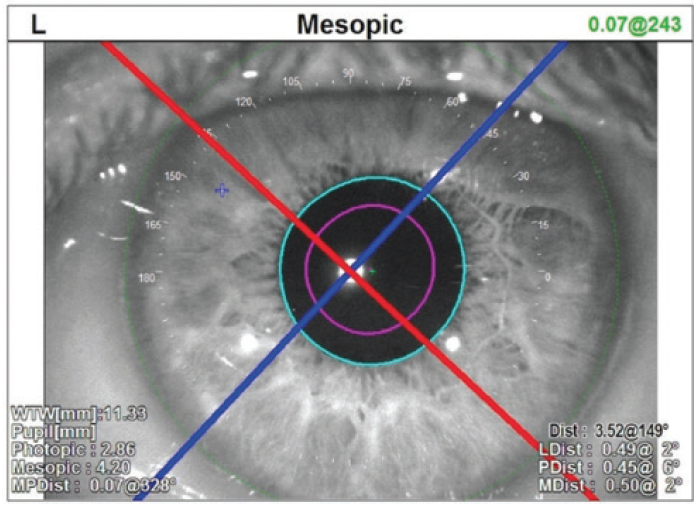
Figure 2. One of the interesting aspects of this case was the patient had a significant angle kappa—the distance between the pupil center and the visual axis.
As cornea measurements become more important, the angle alpha becomes a critical measurement that should be examined. Angle alpha is the difference between the center of the limbus (optical center of the cornea) and the visual axis. This measurement allows a more precise calculation to align the optics of the implant and the optics of the patient’s eye.
In the end, I chose an EDOF lens for this patient. On her 1-week postoperative visit, this high myopic patient’s refraction was -0.50 D sphere. Her uncorrected distance visual acuity was 20/30 +2, partly because of her preexisting myopic macular changes. Her uncorrected intermediate visual acuity was 20/16, and her uncorrected near vision was 20/25. She was absolutely delighted with the outcome and couldn’t wait to undergo surgery for her second eye.
Conclusion
This patient is a perfect example of our need to achieve improvements in refractive outcomes by employing stronger, more viable means of IOL calculations and taking the time to double-check and cross compare our calculations. Having the ability to reduce the risk of refractive surprises with comprehensive optical biometrics leads to better patient education, stronger surgical strategies, and more positive patient outcomes. In our practice we see a lot of dense cataracts that used to require several immersion scans per week. With the IOLMaster 700, we have cut that down dramatically to, at most, one immersion scan per month. This reduction in testing time by our biometrists has made an impactful difference in our patient flow. With more accurate measurements, we are much less likely to have refractive surprises and, therefore, we are able to deliver the best possible results.
1. Lee AC, Qazi MA, Pepose JS. Biometry and intraocular lens power calculation. Curr Opin Ophthalmol. 2008;19(1):13-17.
2. Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368-376.
3. Refractive outcomes in post-myopic LVC eyes–Barrett True K formula with Total Keratometry (TK). zeiss.com https://www.zeiss.com/meditec/int/resource-center/clinical-insights/articles/ophthalmic-diagnostics/refractive-outcomes-in-post-myopic-lvc-eyes.html. Published September 9, 2019. Accessed November 20, 2019.




