Accurate Biometry: One Key Factor in Achieving Successful Outcomes
By Preeya K. Gupta, MD

Refractive cataract surgery has become increasingly popular over the past decade. More and more patients are approaching their physicians requesting premium options to reduce spectacle dependence. The preoperative workup for the refractive cataract surgery patient requires special attention to numerous details: Among them are the health and shape of the ocular surface; other factors that may influence refractive outcomes, such as accurate biometry and astigmatism measurements; and the diagnosis of coexisting retinal disease.
In the workups for all of my refractive cataract surgery patients, I use the following tests routinely, in addition to slit-lamp and fundus examinations: corneal topography, point-of-care tear film testing, and optical and swept-source OCT biometry. Here, each of these are discussed in turn.
CORNEAL TOPOGRAPHY
Astigmatism management is a crucial component of refractive cataract surgery. Corneal topography can be a useful tool to highlight corneal pathology and to ensure that the patient has regular astigmatism. I first look at the pattern of astigmatism. Patients with corneal conditions such as Salzmann nodules, anterior basement membrane dystrophy, or corneal ectasia will have an irregular, nonsymmetric pattern. This should signal the need for additional examination before proceeding with refractive cataract surgery. Often, patients with corneal lesions such as basement membrane dystrophy and Salzmann nodules will benefit from treatment with superficial keratectomy to restore normal corneal shape and regularity before cataract surgery.
Topography can be helpful to identify dry eye disease (DED) as well. Patients with areas of missing data on the map or with a variegated pattern may have DED. Preoperative treatment of DED can help to restore a healthy tear film, resulting in more reliable biometric measurements.
TEAR FILM TESTING
We routinely use a questionnaire to screen patients for undiagnosed DED. In a recent study,1 we found that 80% of patients presenting for cataract surgery have signs of ocular surface dysfunction, yet many are undiagnosed. If we do not screen for DED, it is likely that patients will remain undiagnosed.
A frequent cause for unhappiness after cataract surgery is exacerbation of DED symptoms. Further, untreated DED can have a negative impact on keratometry measurements,2 which may negatively affect refractive outcomes. Two tests that are readily available in clinical practice and easy for technicians to administer are tear osmolarity and testing for the inflammatory biomarker MMP-9.
Results from the screening questionnaire can be used to create a standard protocol for testing patients when DED is suspected. Patients should be treated aggressively before surgery to repair the ocular surface and also to establish for the patient that he or she has two concomitant disease processes: cataract and DED.
BIOMETRY AND OCT
I prefer to use two devices to obtain biometric measures: optical biometry and swept-source OCT biometry. The latest generation of biometric devices, which use swept-source OCT, such as the IOLMaster 700 (Carl Zeiss Meditec), achieve better penetration into dense cataracts, and they can perform foveal OCT and total corneal astigmatism measurement. I look for consistency between these devices, with any inconsistencies preempting further investigation to make sure there are no factors causing fluctuation in measurement, such as DED or corneal irregularity. Total keratometry measurement is a recent addition on the IOLMaster 700. This feature allows more precise correction of astigmatism because it includes measurement of both anterior and posterior corneal astigmatism.
The foveal OCT is also helpful to screen for macular pathology such as epiretinal membrane, macular edema, and age-related macular degeneration, among other posterior segment issues. If something abnormal is identified, our technicians proceed with a full OCT. It is vital to rule out retinal pathology when we offer refractive cataract surgery, as its presence may limit BCVA outcomes.
1. Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44:1090-1096.
2. Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Ptovin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672-1677.
Achieving 20/happy: My Favorite Preoperative Diagnostics for Refractive Cataract Surgery
By Blake K. Williamson, MD, MPH, MS

The preoperative workup is crucial for success in modern cataract surgery. Today, I don’t think surgeons can feel completely confident that they are offering the best for their patients if they are performing only a slit-lamp examination and biometry in their workup routine.
On the other hand, I don’t think it’s necessary to go overboard and purchase three different topographers just so that you can plan a toric IOL implantation. So how does one decide which diagnostics to purchase (while still staying solvent) in today’s arms race of ophthalmology technology?
Different surgeons use different tools, and there is no one formula for success. You need to do what works best for you and your patients. That said, there are some machines that have become gold standards as parts of the preoperative workup, and there are other diagnostic adjuncts that I have found useful for decision-making. This article examines the utility of both kinds of technologies.
ESSENTIALS
There is no replacing a careful history, slit-lamp examination with corneal staining, and accurate biometry. These should be considered the bare minimum for a cataract workup. In many practices, the patient’s history is taken by a scribe or technician, and a practitioner might skip over this and go straight to the eye vitals. This is a mistake, in my opinion. I tend to take the history myself for many cataract workups.
Remember that how you ask certain questions will often change your patient’s answers. If you say: “Have you had eye trauma?” the patient might say no. But ask: “Have you ever hit your head or been in a car wreck?” and maybe the answer changes. And maybe now you’ll look a little more closely for subtle phacodonesis that might have been skipped over previously. And maybe you decide to have a capsular tension ring in the OR for this patient’s surgery. History matters.
The person doing your A-scan biometry also matters. We have the same person in our practice perform all A-scans. She does more than 4,000 per year, and she is fantastic. Designating one person for this task is helpful to ensure the accuracy and reproducibility of your measurements. After all, IOL choice is decided mostly based on axial length and keratometry (K) measurements, so you want these to be as accurate as possible.
GOING TO THE NEXT LEVEL
I believe corneal topography is a crucial part of the modern cataract workup for many reasons. Primarily, it provides a high-definition map of the anterior curvature. But it also can detect comorbidities that may rule in or out certain IOL technologies. For example, I wouldn’t want to place a multifocal IOL in a patient with irregular astigmatism or pellucid marginal degeneration, and it is hard to see those conditions without topography.
Topography is also useful to identify DED, anterior basement membrane dystrophy, and other corneal issues. Finally, it is a helpful second source of K measurements. You want to be sure that the results of topography and biometry match with regard to magnitude and meridian of cylinder; if they don’t, you need to find out why.
Another technology I use in addition to topography is wavefront aberrometry with the OPD-Scan III (Nidek). The device provides wavefront autorefraction and wavefront analysis of the eye’s optical system, including root-mean-square higher-order aberrations and corneal coma, which can assist in IOL selection.
I also think an OCT of the macula is crucial for all cataract workups—even though we still can’t bill for it. No human being can see subtle macular pathology as well as an OCT can. A recent study by surgeons on the retina service at Johns Hopkins University found that OCT was able to identify 10% more epiretinal membranes and 25% more macular edema, and it was 200% more likely to identify vitreomacular traction compared with the authors’ own dilated examinations (Figure).1
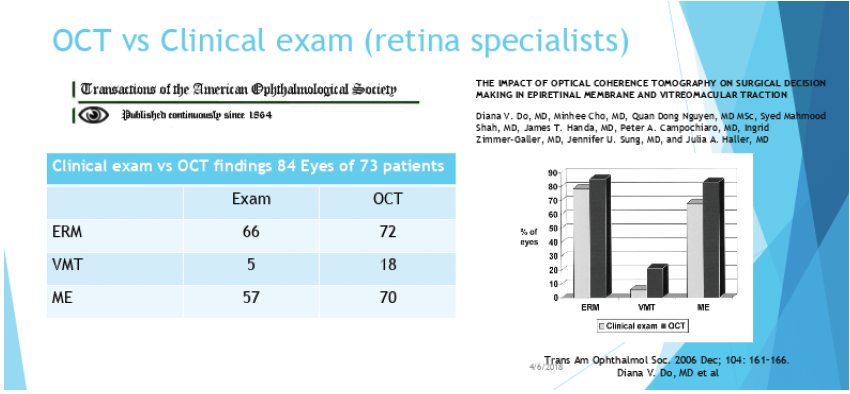
Figure. In one recent study, OCT was able to identify retinal issues more often than trained clinicians. Abbreviation: ERM, epiretinal membrane; VMT, vitreomacular traction; ME, macular edema.
I routinely see subtle parafoveal cysts on OCT that I would not have seen otherwise. This is important because it can affect what IOL I believe the patient is best suited for. OCT allows me to identify patients in need of a retina consultation so that they can be treated and have their eyes prepared for eventual cataract surgery.
If I see patients with good visual potential but subtle epiretinal membranes or vitreomacular adhesions, I may not send them for a retina consult, but I do show them the defect on OCT so that they know they have a preexisting issue that I won’t be correcting and that it will likely prevent them from achieving perfect vision. I routinely use an imperfect macular OCT to dramatically undersell a patient’s potential visual outcome when I know that patient still has good visual potential. That way, I’ll likely over-deliver on expectations.
LAGNIAPPE
In Louisiana, we often use the word lagniappe, which means “a little something extra.” For the purposes of this article, a lagniappe technology is something that might not be as mission-critical to ensure good outcomes as those we have already discussed. But these are technologies I use routinely and would not want to be without.
For example, I like knowing the status of the patient’s tear film, so I find that point-of-care testing for osmolarity and the inflammatory biomarker MMP-9 is helpful in identifying DED patients I may have missed otherwise. These tests sometimes prompt me to start immunomodulatory therapy and postpone surgery until the refractive situation is stable.
I also find the HD Analyzer (Visometrics) to be extremely helpful for evaluating tear breakup time and imaging of the meibomian glands. Poor meibomian gland health may rule out certain IOL technologies or prompt me to initiate our lid physical therapy protocol. This regimen includes thermal pulsation therapy and microblepharoexfoliation, with topography and biometry repeated after 1 month. Many times I’ve seen patients I thought would be toric IOL candidates before this treatment, but afterward they are not—and vice versa.
I occasionally use the iDesign wavefront aberrometer (Johnson & Johnson Vision) to assist in defining the magnitude and meridian of astigmatism if there are differences between the other biometric tests. I also occasionally use full field electroretinography (ffERG, Diopsys) in patients with lenses that are too dense for OCT to penetrate. This allows me to have some idea of their retinal functional outcome before I recommend cataract surgery.
A corneal tomographer is a great tool for defining total corneal power in cataract surgery planning. It is worth noting, however, that the Barrett Toric Calculator, which is accessible on the ASCRS website, attempts to account for total corneal power. This feature is helpful, especially when combined with intraoperative aberrometry.
Finally, widefield fundus imaging has been helpful in screening diabetic patients and high myopes for retinal pathologies.
CONCLUSION
It is important to have an organized, comprehensive preoperative workup routine if you want to deliver 20/happy outcomes in 2019. Your testing should help you rule in or out certain IOLs and give you confidence in your surgical planning.
Further, these technologies will enable you to diagnose many chronic progressive diseases that have no cure and have nothing to do with cataracts, such as primary open-angle glaucoma, age-related macular degeneration, and DED. Positioning these issues as primary problems, and cataracts as something temporary and fixable, will aid in lowering expectations and preventing unhappy patients.
1. Do DV, Cho M, Nguyen QD, et al. The impact of optical coherence tomography on surgical decision making in epiretinal membrane and vitreomacular traction. Trans Am Ophthalmol Soc. 2006;104:161-166.




