CASE PRESENTATION
A 57-year-old woman presents with a complaint of blurred near vision after planned monovision refractive cataract surgery.
The patient’s past ocular history is significant for bilateral LASIK performed in 2004, when she was 45 years of age. Her original pre-LASIK refraction was -4.00 OD and -3.50 -0.50 × 180 OS, correctable to 20/20 OU. The patient opted for planned monovision with a -1.25 D target in her left eye. After the original LASIK surgery, she enjoyed 20/20 distance and near vision with both eyes working together (Figure 1).
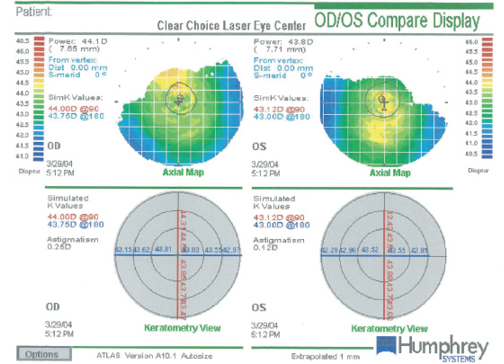
Figure 1. Evaluation of both of the patient’s eyes with the Atlas 9000 Corneal Topography System (Carl Zeiss Meditec) shows a regular appearance of the virgin corneas and an absence of apparent pathology prior to LASIK.
Twelve years later, the patient had blurred vision in the left eye, with a loss of both distance and near vision and a BCVA of 20/50 (-1.00 D OS). She was diagnosed with 2+ nuclear sclerotic and cortical cataracts. Her near UCVA was 20/60 OS. The patient’s right eye had trace nuclear cataracts, a distance UCVA of 20/20, and a near UCVA of 20/70. The risks and benefits of and the alternatives to cataract surgery for the left eye were discussed with the patient, and she chose to undergo cataract surgery with a monofocal IOL to target near vision (Figures 2 and 3).
Successfully performed in June 2016, the procedure achieved a refractive target of -1.25 D. The surgeon, guided by intraoperative aberrometry, implanted a Tecnis ZCBOO IOL (Johnson & Johnson Vision).
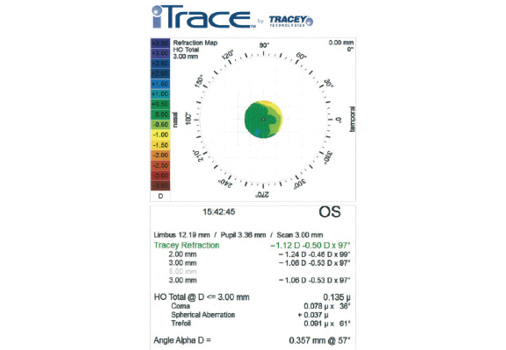
Figure 2. As expected, a refraction map of the left eye with the iTrace (Tracey Technologies) obtained after LASIK but before cataract surgery reveals mild myopia. Note that the spherical aberration is +0.37 µm and the total higher-order aberrations measure 0.135 µm.
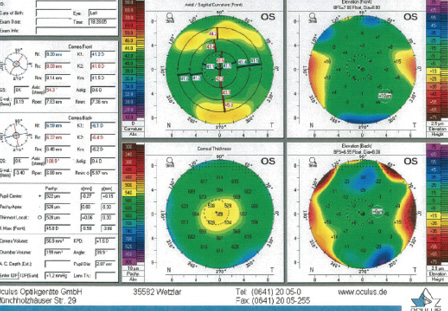
Figure 3. Imaging of the left eye with the Pentacam (Oculus Optikgeräte) shows a total corneal thickness and normal appearance with central flattening that are consistent with a postmyopic LASIK treatment. There are no signs of abnormal pathology.
Soon after cataract surgery, the patient began to complain of poor near visual function: “I had monovision with LASIK before, and I feel my vision after cataract surgery is not nearly as good as it was after LASIK.” After ample time to adapt to her new vision status, the patient had no improvement in function, so the surgeon offered to demonstrate additional myopia with contact lenses. The physician explained to the patient that, if a monovision trial with more near power were successful, a secondary surgical intervention mimicking the appropriate add power could be considered. Various lenses with differing amounts of add power were given to the patient, but she was not successful in achieving functional near vision without the induction of symptomatic anisometropia.
What management would you recommend for the left eye at this point in time? What is your typical approach to patients with a history of successful LASIK monovision who subsequently present with cataracts? Would you have recommended a different approach for the first cataract surgery, a different refractive target, and/or an alternative IOL?
—Case prepared by William F. Wiley, MD.

ROBERT J. WEINSTOCK, MD
All refractive surgeons encounter this challenging situation. Despite our best efforts, we just cannot make some patients happy. Sometimes, it is because a specific visual goal has not been met, and sometimes, the patient’s high expectations are the reason. Regardless, it is prudent not to chase problems that may not have an easy solution but carry additional risk for both the patient and the surgeon.
Upon reading through this case, my gut instinct was to suggest thoroughly explaining to the patient that the objective refractive targeting goals were met but that her expectations might have been set too high. Because a more anisometropic monovision trial was not successful, there are few easy options for making the patient happy. Oftentimes, in these cases, I offer to refund what the patient spent out of pocket on noncovered refractive services, which tends to soften the blow to the patient that his or her desired visual outcome could not be achieved.
In hindsight, the only thing I might have done differently would have been to implant a monofocal IOL with no induced spherical aberration such as the LI61AO, Akreos, or even the Crystalens (all from Bausch + Lomb). This strategy might have improved the patient’s depth of field, owing to the positive spherical aberration present on the iTrace analysis of her cornea. The Tecnis lens has negative spherical aberration, which cancels out the positive amount on the cornea and leaves an optical system with no depth of field.
In addition, I tend to target closer to -2.00 D in all my monovision IOL patients. I find this refraction to be almost universally accepted if the patient has a history of successful monovision in contact lenses or with LASIK. It ensures that he or she will have functional computer and reading vision under most conditions. In this case, however, the risk-benefit ratio for a lens exchange and improved near vision with a similar -1.25 D target does not make sense to me. This patient likely had some residual accommodation in addition to her -1.25 D target after LASIK, which is another reason why her reading vision was better after that procedure than it is with the IOL. Perhaps the Crystalens has the best theoretical chance of delivering the outcome that this patient desires without making her more anisometropic.

ELIZABETH YEU, MD
Pseudophakic monovision is an excellent option for patients who were successful with monovision (ie, laser vision correction or contact lenses) prior to cataract surgery. This situation is unique because the patient is relatively young, and because the -1.25 D of myopia in her left eye was adequate for her near needs while she was in her 40s and early 50s when combined with natural accommodation. Had the cataract formed when she was in her 60s, the patient would have better understood that her -1.25 D monovision status would have provided intermediate vision at best, without useful near vision.
The Tecnis ZCB00 is a wonderful monofocal IOL choice for a patient who is status post myopic LASIK, because the lens has the most negative spherical aberration of the aspheric IOLs, -0.27 µm, which can neutralize the positive spherical aberration that her cornea likely possesses.1 Although the ZCB00 provides an excellent overall quality of vision, it may have reduced any corneal pseudoaccommodation that the patient has had by neutralizing the spherical aberration. In reality, though, I do not think any monofocal IOL, even one that could have increased the patient’s spherical aberration, would have resulted in a more meaningful accommodative potential or led to a different functional outcome.
A laser refractive enhancement to increase the myopia is not an option in this case, as demonstrated by the contact lens trial. The options for this patient are topical pilocarpine 1%, a Kamra corneal inlay (AcuFocus), or an IOL exchange with an extended-depth-of-focus (EDOF) IOL. The pilocarpine might or might not work, and the periorbital headache might rule out this strategy. The patient’s current level of myopia is in the sweet spot for the Kamra. That said, I have been extremely pleased with the EDOF IOL for a mini-monovision status (goal -0.50 to -1.00 D) in patients who were already successful with monovision prior to the surgery. Although night vision symptoms with the EDOF IOL will be amplified by residual myopia, monovision patients have already demonstrated an ability to suppress night vision symptoms. This particular patient has a well-centered ablation bed, based on the diagnostics provided, and an IOL exchange for an EDOF IOL with a refractive goal of -0.75 to -1.25 D would likely produce a highly satisfactory outcome.

WHAT I DID: WILLIAM F. WILEY, MD
Recent advances in refractive surgery have broadened its reach. Monovision has been a successful approach to achieving both distance and near vision for many presbyopic LASIK patients. That said, monovision LASIK is not a perfect solution, because it requires certain compromises. In particular, as the eye ages and presbyopia increases, the patient develops an increased need for near vision. In short, monovision LASIK is a static solution to a dynamic problem.
Monovision LASIK patients pose a specific set of challenges when they present with cataracts. Many had success with monovision while they retained a certain baseline of accommodation. Because a monofocal IOL yields no accommodation, however, they may not attain the same result in a monofocal pseudophakic state. The functional range of vision in a monofocal pseudophakic eye is less than in a phakic eye, because the latter has some baseline amplitude of accommodation.
A multifocal lens can be considered in a situation like this, but again, the technology comes with compromises. Typically, patients sacrifice some quality of vision (more so in nighttime conditions) with a multifocal compared with a monofocal lens. This loss of quality can be more dramatic in an eye that previously underwent corneal refractive surgery.
After thorough consideration of the patient’s visual goals, I decided that she might benefit from an aperture corneal inlay. These devices work best when applied to a baseline myopic refraction. My expectation was an increase in both distance and near UCVA while maintaining the good intermediate vision provided by the -1.25 D refractive state. I thought the aperture inlay for this mildly myopic pseudophakic patient would best simulate the visual function she benefited from as a phakic myopic LASIK patient. After discussing the risks, benefits, and alternatives, we proceeded to surgery.
Using a femtosecond laser, I created a corneal tunnel at a depth of 285 µm. In my experience, deep tunnels are more biocompatible with aperture corneal inlays. In this case, the deep tunnel allowed me to place the inlay below the previously created LASIK flap.
The patient tolerated the procedure quite well and noticed an improvement in her near and distance visual function. Objectively, her distance UCVA improved to 20/40 OS, while her near UCVA improved to 20/25 in that eye.
1. Yeu E, Wang L, Koch DD. The effect of corneal wavefront aberrations on corneal pseudoaccommodation. Am J Ophthalmol. 2012;153(5):972-981.




