
Pharma has played a major role in how physicians care for and treat patients with ophthalmic disease. In recent years, the conduct and critical analysis demonstrated by FDA trials have brought excellent medications to market. Clinicians now have more effective and safer antibiotics, anti-inflammatory agents, and drugs for glaucoma and dry eye disease than ever before.
By participating in more than 50 phase 2 through phase 4 trials, my practice has been at the forefront of this trusted system. I admire the time-honored scientific empirical method and the well-organized system that allows the FDA to conduct its trials. I acknowledge and respect the hard work of established pharmaceutical players such as Alcon, Allergan, and Bausch + Lomb. I encourage them to continue their efforts to create new therapies for tomorrow.
AT A GLANCE
• Compounded medication offers the opportunity for disruptive technology that addresses the burdens of cost and compliance.
• Just as big pharma has performed empirical studies to prove their new formulations, it is incumbent on the manufacturers of compounded medication to prove the value and safety of theirs.
• Ocular Science just completed a US multicenter, randomized, observer-masked, prospective study comparing its preservative-free Omni with current multidrop therapy. The compounded combination medication was safe and therapeutically equivalent to the conventional use of multiple medications for controlling inflammation after cataract surgery.
Despite major strides, however, cost and compliance remain substantial challenges for patients.1-3 Just as Tesla, Dollar Shave Club, and Uber created a paradigm shift in the automotive, shaving, and transportation industries, respectively, compounded medication offers the opportunity for disruptive technology. This innovative approach to prescription medication addresses the burdens of cost and compliance, in turn increasing efficacy. Postoperative drop solutions can carry the antibiotic, steroid, and nonsteroidal anti-inflammatory drug in one bottle that patients use a few times a day, making dosing significantly easier.
AN EXAMPLE
For surgeons who want to potentially reduce postoperative drops to just a topical nonsteroidal anti-inflammatory drug, Ocular Science has formulated intracameral combinations such as dexamethasone and moxifloxacin. The 2013 Kaiser study by Shorstein and colleagues firmly established the safety of an intracameral antibiotic by showing the rate of endophthalmitis to be lower than with postoperative drops.4
Although there are many early adopters of compounded medications, other ophthalmologists have been sitting on the sidelines. These doctors recognize the simplicity of compounded medications and appreciate the cost savings, but they question the drugs’ safety and efficacy. For that reason, just as big pharma has performed empirical studies to prove their new formulations, it is incumbent on the manufacturers of compounded medication such as Ocular Science and Imprimis to prove the value and safety of theirs.
To that point, Ocular Science just completed a US multicenter, randomized, observer-masked, prospective study comparing its preservative-free Omni with current multidrop therapy. Omni combines prednisolone sodium phosphate 1%, moxifloxacin hydrochloride 0.5%, and ketorolac tromethamine 0.5%. The study enrolled 80 patients, who underwent unilateral, uncomplicated, extracapsular phacoemulsification with implantation of a posterior chamber IOL. Forty patients administered Omni four times daily for 2 weeks, then twice daily for 2 weeks. In the other treatment arm, 40 patients instilled moxifloxacin hydrochloride ophthalmic solution 0.5% (Vigamox; Alcon) and prednisolone acetate 1% (Pred Forte; Allergan) four times daily for 2 weeks, then twice daily for 2 weeks, and nepafenac ophthalmic suspension (Ilevro; Alcon) once daily for 4 weeks. The primary endpoint was to investigate resolved ocular inflammation after cataract surgery. The secondary endpoint was the reduction of pain after surgery. The investigators will also assess corneal staining and evaluate subclinical and clinical cystoid macular edema using spectral-domain macular optical coherence tomography.
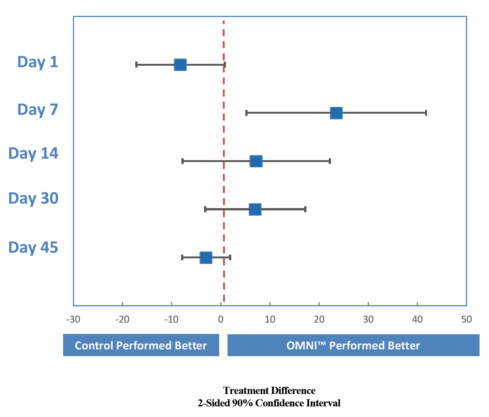
Figure. Omni demonstrated a higher SOIS = 0 at days 7, 14, and 30 after cataract surgery.
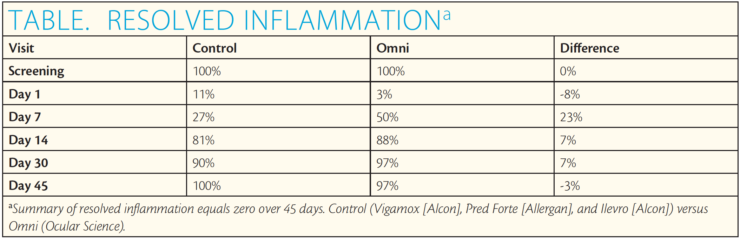
The Table and Figure summarize the results. The data at day 7 indicate that 50% of Omni patients had total inflammatory clearance (summary of resolved inflammation [SOIS] = 0) compared with 27% of the patients using brand-name drops. That is a 23% higher clearance rate for Omni. At day 14, 88% of Omni patients versus 81% of controls had an SOIS of 0. As expected in an active-controlled study, none of the P values (in the table’s difference column) was below 0.05 (the typical alpha level to denote nominal statistical significance). The P values must be interpreted carefully. The confidence intervals (and the graph of them) really demonstrate the similarity or noninferiority of Omni compared with the control. In terms of pain reduction, corneal staining, and macular thickness as measured with optical coherence tomography, Omni performed similarly to the control.
In this clinical study, a compounded combination medication was safe and therapeutically equivalent to the conventional use of multiple medications for controlling inflammation after cataract surgery.
A LOOK AHEAD
Over the next few quarters, Ocular Science will present more prospective studies on its postoperative combinations for LASIK and glaucoma to establish confidence in these medications’ safety and efficacy. In addition, the company will present the retrospective results of its novel dry eye therapy, Genesis, an amniotic cytokine extract, at the American Society of Cataract and Refractive Surgery Congress & Symposium this month. The company maintains that combination compounded medication, when proven through the scientific method, will provide a new class of treatment for patients.
1. Kholdebarin R, Campbell RJ, Jin Y-P, Buys YM, Canadian Compliance Study Group. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008;43(4):454-456.
2. Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74:477-480.
3. Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Clin Ther. 2003;25:8:2307-2335.
4. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39(1):8-14.




