CASE PRESENTATION
A 42-year-old white man is referred to the Duke University Eye Center Cornea Service for a central corneal ulcer with a hypopyon in his right eye. The patient sustained the ocular injury while mowing the lawn, with debris getting into the eye while he was wearing contact lenses. He was diagnosed with culture-positive Fusarium species by the referring ophthalmologist and was treated with oral voriconazole 200 mg twice daily and frequent topical natamycin 5% and voriconazole 10 mg/mL. The patient underwent epithelium-off corneal collagen cross-linking (CXL) approximately 4 weeks after diagnosis of the ulcer and was treated with a loteprednol steroid taper after the procedure. His condition subsequently progressed, with increasing eye pain, a nonhealing epithelial defect, and a worsening corneal infiltrate.
Upon evaluation, the patient has a large corneal infiltrate with necrotic stroma, which is approaching the limbus, and a hypopyon (Figure 1). His UCVA measures 20/70-1. B-scan ultrasound of the right eye shows no evidence of posterior segment involvement. Reculturing of the corneal infiltrate is negative for bacteria, fungus, and Acanthamoeba. Confocal microscopy reveals no evidence of hyphae or cysts. Given the worsened infiltrate with broad antifungal and antibacterial coverage and its proximity to the corneal limbus, the patient undergoes a therapeutic penetrating keratoplasty (PKP).
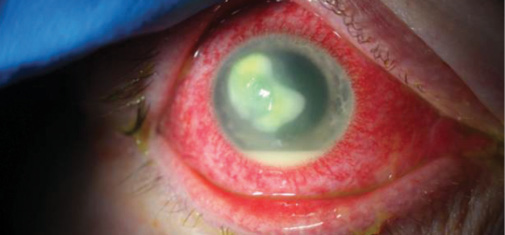
Figure 1. Initial evaluation of the eye with a Fusarium corneal infiltrate and hypopyon.
After surgery, the patient is maintained on oral voriconazole, topical natamycin 5%, and topical amphotericin B 1.5 mg /mL. Pathology of the host cornea reveals diffuse fungal penetration through Descemet membrane into the anterior chamber. The postoperative course is complicated by a recurrence of the infiltrate in the corneal graft. This is treated with subconjunctival and intracameral injections of voriconazole (1 mg/mL) administered once weekly on average as well as two intracameral voriconazole injections performed over the next 11 weeks. Resulting corneal neovascularization is treated with topical tacrolimus 0.3% ophthalmic solution. After discussion with an infectious diseases specialist, polyhexamethylene biguanide 0.02% ophthalmic solution and voriconazole 10 mg/mL ophthalmic solution are added, and notable consolidation in the fungal infection is visible on clinical examination.
The patient’s UCVA gradually improves to 20/70, with a pinhole visual acuity of 20/40. Scarring and deep neovascularization are present on examination (Figure 2). A repeat PKP to improve visual acuity is planned once the infection and associated inflammation completely resolve.
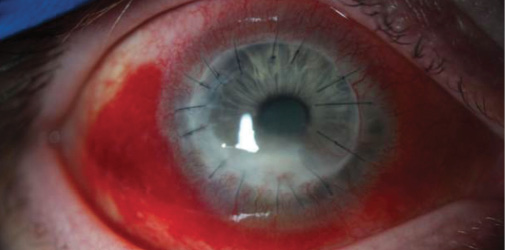
Figure 2. The same eye 3 months after PKP with corneal neovascularization and an inferior fungal infiltrate.
With the recent FDA approval of the KXL System (Avedro) and the anticipated usage in refractive surgery, what additional role will CXL offer in the management of active microbial keratitis? What are possible unintended consequences that may develop from the limitations in treatment depth or tissue changes that may affect antimicrobial penetration?
—Case prepared by Ashiyana Nariani, MD, MPH;
Gargi Khare Vora, MD; and Alan N. Carlson, MD.

MINAS CORONEO, AO, BSc(Med), MBBS, MSc Syd, MD, MS unsw, FRACS, FRANZCO
From the perspective of a Monday morning quarterback, I would list five concerns in this case. First, it appears that the initial and subsequent treatments were suboptimal, which can be a factor in eye loss. It is likely that the infection was deep-seated, and repeated intrastromal voriconazole (with or without debridement) is considered to be more effective than topical treatment to get high tissue levels of antifungal treatment.1,2
Second, in a setting of recalcitrant disease, molecular pathogen identification and in vitro antifungal susceptibility testing can guide treatment.3 Other antifungal treatments such as intravenous micafungin could have been considered, because voriconazole-resistant cases may respond.4
Third, photoactivated chromophore for infectious keratitis (PACK) CXL effects are limited to the anterior cornea, so the procedure may not be suitable for cases of filamentary fungal keratitis where there is deep penetration into the stroma. If the Dresden protocol was used, PACK-CXL might have been suboptimal.5
Fourth, prior to PACK-CXL, imaging with confocal microscopy or anterior segment optical coherence tomography might have indicated the depth of the infection and dictated a different approach.6
Fifth, unless the limbus was protected, it is possible that damage (with evidence of corneal neovascularization) will compromise the outcome of future graft procedures. The use of topical steroids and tacrolimus may not have been helpful.
Apart from limbal and possible endothelial damage and reactivation of herpes simplex virus, other potential unintended consequences of PACK-CXL are accelerated corneal thinning and corneal perforation, particularly where there is a significant inflammatory tissue response.6
Patients’ and surgeons’ improved access to PACK-CXL requires the development of better guidelines derived from rigorous studies.

RAJESH FOGLA, DNB, FRCS(Edin), MMed(Ophth)
Managing fungal keratitis from filamentous species (Fusarium) is clinically challenging. A limited response to antifungal agents and rapid progression often necessitate surgical intervention. I would have preferred to perform a therapeutic PKP in this case instead of a CXL procedure. Although some positive effect of CXL for bacterial keratitis has been noted in published studies, evidence of its effectiveness for fungal keratitis is lacking.
CXL is supposed to help by (1) increasing tissue resistance to enzymatic digestion, thereby reducing corneal melting, and (2) offering the antimicrobial effect of free radical production. This action is limited to the anterior one-third of the cornea, however, so in fungal keratitis, the deeper location of fungal infiltrates can reduce the effectiveness of CXL. Antifungal drugs have poor corneal penetration, an issue potentially exacerbated by CXL, which tends to induce structural changes in the corneal stroma, making it more compact. This could explain the worsening keratitis after CXL in this case. Steroids are usually not recommended in fungal keratitis, and their use after CXL could also be responsible for the worsening keratitis.
Currently, CXL seems to hold promise for the treatment of superficial infectious keratitis, especially bacterial keratitis. Further research with randomized clinical trials is necessary to establish the procedure’s efficacy for this indication.

WILLIAM B. TRATTLER, MD
The FDA’s approval of CXL made an important therapeutic treatment widely available to US patients. In addition to its role for the treatment of keratoconus and post-LASIK ectasia, CXL can help patients with ectatic conditions such as pellucid marginal degeneration, ectasia following radial keratotomy or astigmatic keratotomy, and recurrent keratoconus after corneal transplantation. CXL also appears to be an effective adjunctive therapy for patients with microbial keratitis. Moreover, studies suggest that CXL can help with early bacterial corneal ulcers and that it may have a role in the management of fungal corneal ulcers.5
Despite CXL’s promise, surgeons must understand its limitations. In particular, the procedure is not effective for microbial keratitis that is deep within the cornea, because the depth of effect is limited. In the case described herein, CXL was employed early in the patient’s course. At the time, however, the Fusarium infection was already full thickness, so the CXL procedure could not effectively eliminate the fungal infection from the cornea.
On the positive side, there is evidence that CXL can play an adjunctive role in the therapy of bacterial, fungal, and potentially even Acanthamoeba keratitis.5 If CXL is used for infectious keratitis, the patient should continue appropriate antimicrobial therapy. Perhaps in the future, innovations in the CXL procedure will allow a single treatment to eliminate certain corneal infections. In the meantime, however, clinicians who employ CXL for infectious keratitis should do so with the understanding that the procedure can reduce the load of micro-organisms but, by itself, may not eliminate the infection.



ASHIYANA NARIANI, MD, MPH; GARGI KHARE VORA, MD; AND ALAN N. CARLSON, MD
Drs. Coroneo, Fogla, and Trattler have done a wonderful job sharing expertise that they have accumulated over a lengthy period of time, while US surgeons awaited access to CXL. It is important to recognize the limitations of this procedure regarding the depth of therapeutic treatment of corneal infection. What remains uncertain is whether or not CXL alters corneal tissue in a manner that contributes an additional barrier to antimicrobial penetration that could make the treatment of infectious microbial keratitis more challenging.
1. Prakash G, Sharma N, Goel M et al. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146:56-59.
2. Guber I, Bergin C, Majo F. Repeated intrastromal injections of voriconazole in combination with corneal debridement for recalcitrant fungal keratitis - a case series [in German]. Klin Monbl Augenheilkd. 2016;233:369-372.
3. Taylan Sekeroglu H, Erdem E, Yagmur M, et al. Successful medical management of recalcitrant Fusarium solani keratitis: molecular identification and susceptibility patterns. Mycopathologia. 2012;174:233-237.
4. Niki M, Eguchi H, Hayashi Y, et al. Ineffectiveness of intrastromal voriconazole for filamentous fungal keratitis. Clin Ophthalmol. 2014;8:1075-1079.
5. Tabibian D, Mazzotta C, Hafezi F. PACK-CXL: corneal cross-linking in infectious keratitis. Eye Vis (Lond). 2016;3:11.
6. Abbouda A, Estrada AV, Rodriguez AE, et al. Anterior segment optical coherence tomography in evaluation of severe fungal keratitis infections treated by corneal cross linking. Eur J Ophthalmol. 2014;24:320-324.




