
Thank goodness for the innovators! As an ophthalmology resident (circa 1994), I recall reading a blurb from a survey in a bimonthly publication. A practicing ophthalmologist stated that he simply did not see the IOL world’s getting any better than it was with an IOL featuring a 6-mm PMMA optic. Although I implanted these lenses, and they did offer great vision, I was dumbfounded by that doctor’s remark. Foldable IOL technology was evolving, and decreasing the incision size to 3 mm from 6 mm seemed very appealing for the physical integrity of a globe at the least, quick healing, and—I hoped—minimizing or eliminating the surgical induction of astigmatism. At the time, we had yet to see toric IOLs, had not heard much about multifocal IOLs, and had not even dreamed someone would try to create an accommodating IOL.
As we venture into 2017, ophthalmology and specifically IOL technology are poised to make another great transition. Thanks to innovators who are not satisfied with the status quo, we as an industry and field of medical endeavor can be very excited. This article takes a look at four technologies that may be available in the not-too-distant future inside or outside the United States.
LIGHT ADJUSTABLE LENS
The Light Adjustable Lens (LAL; Calhoun Vision) comes to ophthalmology from the pioneering work of Dan Schwartz, MD, and his collaboration with 2005 Nobel Laureate in Chemistry Robert Grubbs, PhD. The concept behind the LAL is that spherocylindrical power modification of the IOL after implantation can overcome the inaccuracies of preoperative biometry, predictive IOL formulae, IOL power inaccuracy, healing responses, and difference in the IOL’s final position compared to formulae expectation by allowing these processes to play out and stabilize. Historically, even the best surgeons on average can achieve ±0.50 D from the spherical target, 0.50 D or less of the intended target of cylindrical correction, and ±0.50 D of the spherical equivalent target in approximately 50% of eyes. With intraoperative biometry, reports of 70% of eyes at ±0.50 D from the spherical equivalent target have been made.1 With the LAL (Figure 1), I was able to achieve these same parameters in 93% of eyes with preoperative keratometric cylinder of between 0.75 and 2.00 D.
Figure 1. The LAL.

Figure 2. The FluidVision IOL. A 6-mm central optic is surrounded by chambers filled with isorefractive index silicone oil that is transferred to the optic for near vision and away from the optic for distance vision.
FluidVision IOL
The FluidVision IOL (PowerVision) incorporates a novel design with a 6-mm central optic surrounded by a peripheral reservoir that is filled with isorefractive index silicone oil (Figure 2). The hydrophobic acrylic lens is placed in the capsular bag. Upon the accommodative response, the fluid is displaced centrally into the lens, thereby expanding the central membrane of the optic and facilitating near vision, with reports of an average of 4.00 D of accommodative amplitude, according to the company’s website. With relaxation of the ciliary muscle, fluid returns to the periphery, and distance vision is created. The lens has been studied outside the United States, with accommodation reportedly lasting up to 3 years postoperatively. The company hopes to initiate FDA trials in the next 2 years.
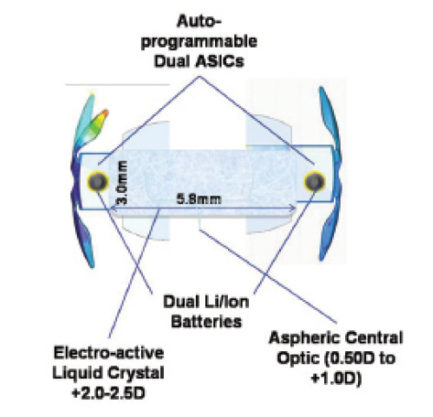
Figure 3. The Sapphire IOL consists of an electroactive liquid crystal, electronics, and lithium ion power cells, which are hermetically sealed in a glass wafer and surrounded by an acrylic outer component.
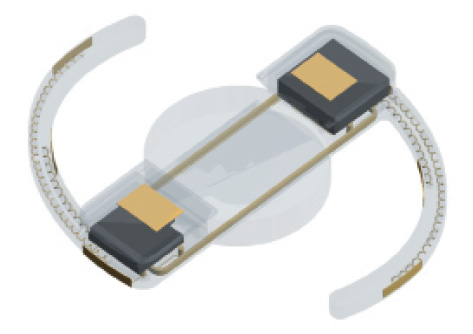
Figure 4. The VistaLens.
SAPPHIRE AUTOFOCAL AND VISTA OCULAR IOLs
At least two electromechanical IOLs are in development. The advantages of these lenses is that they do not require axial movement of the optic to achieve accommodative effect. Electro-optical lens designs attempt to “outsmart” the biological system. If ever there were a cyborg-like concept for a focusing eye, these devices fit the bill. Two companies are attempting to bring this technology to market: Elenza with the Sapphire AutoFocal IOL and Vista Ocular with the VistaLens.
The Sapphire Autofocal IOL has a liquid crystal optic that automatically changes optical power in response to the change in pupillary size for the near triad (Figure 3). When the pupillary photosensors are triggered on near response, the stored charge from the photovoltaic cells adjust the optic for near vision. The power cells are rechargeable via encapsulated microcoils, which also function for two-way communication with the device and external programming. The liquid crystal, electronics, and power cells are hermetically sealed in a glass wafer surrounded by an acrylic outer component. Recharging the power cells can be achieved at night by the patient’s wearing a mask over the orbits. An FDA trial is planned to begin in early 2017.
WATCH IT NOW
John Doane, MD, speaks with Mark Kontos, MD, regarding the challenges ophthalmologists face with regard to meeting patients’ expectations.
Vista Ocular is developing implant technologies that bypass both pupillary and ciliary muscle movement and harness the muscle’s action potential to serve as the signal for accommodation. The technology is patented (US patent 8,574,295), and more patents are pending. This design also incorporates rechargeable battery power with a flexible lens system to change optical power in a variable fashion. The company is currently in the process of testing in vivo a sensor system for the detection of the ciliary muscle’s action potential (Figure 4).
1. Lindstrom RL. Results of real-world clinical use of intraoperative aberrometry system with streaming refractive data. Paper presented at: ASCRS/ASOA Symposium & Congress; April 28, 2014; Boston, MA.




