
Corneal collagen cross-linking (CXL; not approved in the United States) has been used in clinical practice for more than 15 years now, and in this time, it has been shown to be an effective approach for stabilizing keratoconus. Occasionally, however, unexpected corneal responses to CXL can occur—even in the hands of the most experienced surgeons—calling into question our understanding of the safety of the procedure and its various protocols.
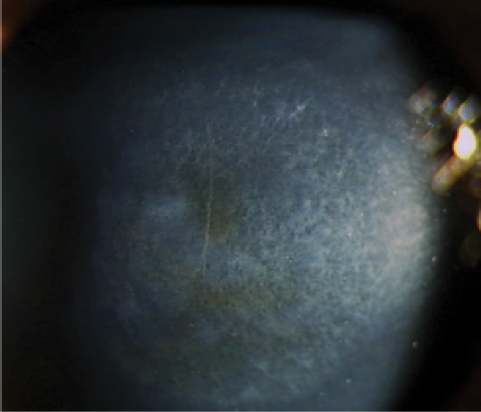
Figure 1. Corneal haze is mediated by activated keratocytes and is usually mild after CXL.
It is my impression that unexpected corneal responses to CXL occur for one of two reasons. First, given that we have less than 2 decades of experience and data with CXL, an unexpected response could indicate that we do not yet fully understand all of the intricacies of the procedure. Second, an unexpected response may result if, under certain conditions, we fail to apply the appropriate knowledge and, thus, disrespect the limits of CXL.
This article provides an overview of unexpected corneal responses to CXL, including corneal haze and remodeling, endothelial and stem cell damage, and infectious keratitis and corneal melt. An improved understanding of these responses and their underlying mechanisms is required to truly evaluate the limits and, thus, the safety of this treatment.
At a Glance
• Unexpected corneal responses to CXL may occur because the intricacies of the procedure are not yet fully understood or because the surgeon disrespects the limitations of CXL.
• Such unexpected responses include corneal haze and remodeling, endothelial and stem cell damage, and infectious keratitis and corneal melt.
• Oxygen is an essential component of CXL for keratoconus, because the cross-linking process implicates the generation of free radicals.
• CXL and PACK-CXL seem to be safe procedures with relatively few surprising results when the limitations of the methods are respected.
HAZE AND REMODELING
Corneal haze and remodeling are related to ultraviolet A (UVA) light and the wound-healing process, as UVA light may induce limbal stem cell and endothelial damage. Haze occurs after CXL and surface ablation. It is mediated by activated keratocytes and is usually mild after CXL (Figure 1). What is interesting is that, under certain conditions, haze does not go away after 3 to 4 months; it is sometimes persistent in patients treated with CXL.
We know that haze is linked to the corneal remodeling process. The Dresden follow-up study showed changes in keratometry (K) readings and remodeling of the corneal surface after CXL.1 Surgeons who perform CXL are likely familiar with the topography maps shown in Figure 2. With roughly 2 years between the pre- and postoperative maps, the difference map shows clear corneal flattening of up to 2.00 D. This is also linked to a haze that is transient and deeper than the haze observed after PRK, going down to the treatment limits of 300 μm.
It is also possible for a massive remodeling effect to occur after CXL.2 Figure 3 shows the topography maps of a patient 4 weeks after conventional epithelium-off (epi-off) CXL, in which UV radiation of 3 mW/cm2 was applied for 30 minutes. A strong reduction in steepest keratometry (Kmax) of up to 9.50 D can be observed in the difference map. The massive remodeling effect shown in Figure 3 was also accompanied by the formation of deep stromal haze (Figure 4).
Compared with the transient haze that typically occurs after PRK and CXL (Table), it seems that, after CXL with massive corneal remodeling, there is a form of haze that may be permanent. This raises the question of whether there is more than one remodeling pathway. This is just one of the issues we do not understand that may lead to an unexpected response after CXL.
ENDOTHELIAL DAMAGE
As previously mentioned, UV light has been shown to have the potential to induce damage to the endothelium. In the 1990s, in vitro studies in rabbit and porcine eyes established a cytotoxicity threshold for the endothelium at 0.36 mW/cm2. A more recent study by Koppen and colleagues redefined this threshold, but it is still quite low.3
Many reports in the literature to date citing complications after CXL used riboflavin solution with dextrane; we now know that dextrane thins the cornea. Further, most of these reports failed to measure corneal thickness prior to irradiation, right after riboflavin installation, which is crucial. This raises questions of pure physics and whether we are respecting the associated parameters derived from Lambert-Beer’s Law.4
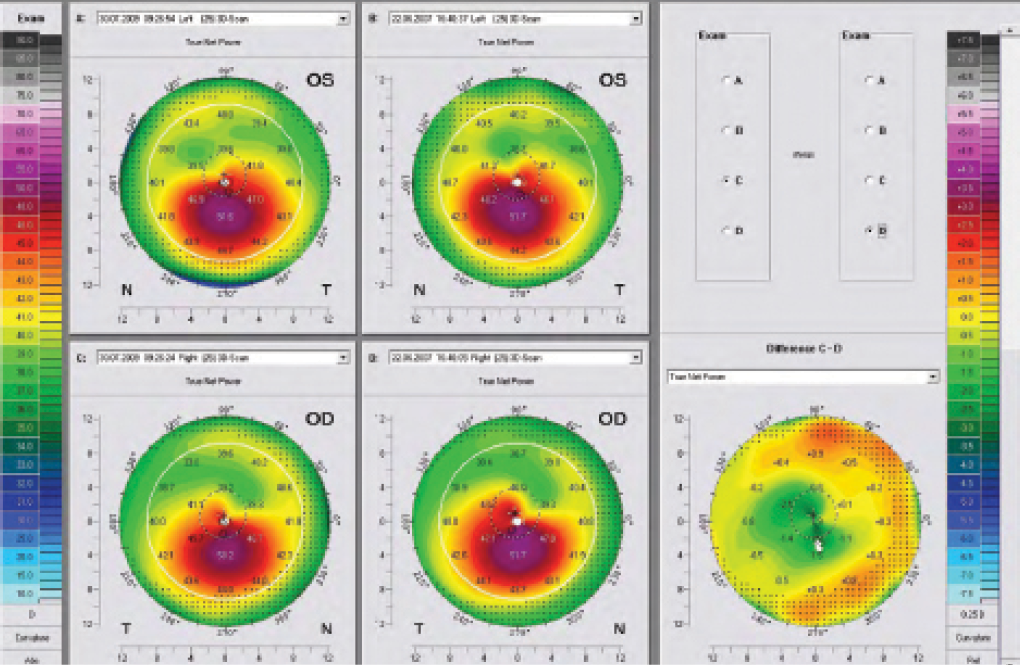
Figure 2. With roughly 2 years between pre- and postoperative maps, the difference map shows corneal flattening of 1.00 to 2.00 D following CXL.
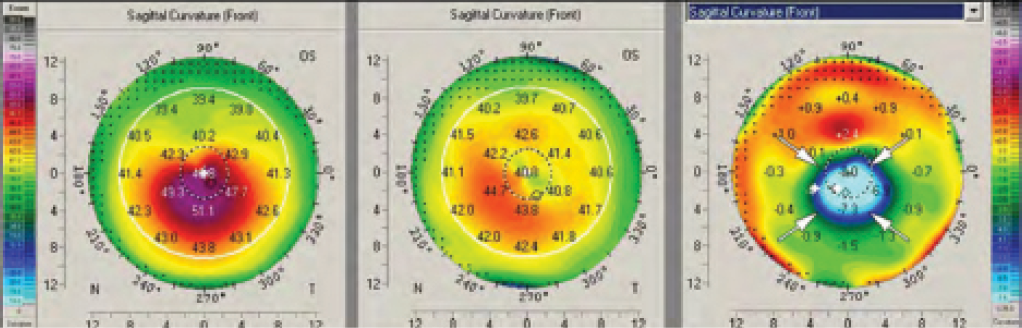
Figure 3. A massive corneal remodeling effect after CXL. A strong reduction in Kmax readings of up to 9.50 D can be observed in the difference map.
STEM CELL DAMAGE
UV light has also been shown to result in damage to stem cells. In some cases, such as in patients with pellucid marginal degeneration or Terrien marginal degeneration, decentered irradiation is clinically necessary; however, this approach has caused us to question whether we harm the stem cells of the limbus when we perform peripheral CXL.
In an experimental setup, my colleagues and I performed epi-off CXL in male New Zealand white rabbits with two irradiation diameters (7-mm central cornea and 13-mm cornea and limbus) using standard and double-standard fluences (5.4 and 10.8 J/cm2, respectively).5 We found that, for both irradiation diameters and fluences tested, there were no signs of endothelial damage, and the time to re-epithelialization was similar to that in untreated controls. Further, histologic and immunohistochemical analysis revealed no differences in the expression of stem cell markers.5
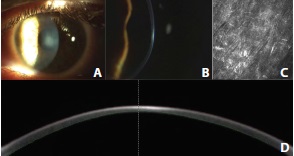
Figure 4. Slit-lamp photographs of central deep stromal haze in a patient’s left eye (A, B). Corneal confocal microscopic section of the anterior corneal stroma at 170 μm. The stroma shows activated keratocytes and hyperreflective deposits corresponding to subepithelial fibrosis (C). High-resolution Scheimpflug imaging of the corneal haze (D).
Even when using fluence that is twice as high as that used in current clinical settings, circumferential UVA irradiation of the corneal limbus did not alter the regenerative capacity of the limbal epithelial cells. In 15 years of clinical CXL use, there have been no reported cases of stem cell decompensation. Thus, it is safe to conclude that we surgeons do not harm the stem cells of the limbus when we perform peripheral CXL and that decentered sector irradiation, as in patients with pellucid marginal degeneration, should be possible without harm.
INFECTIOUS KERATITIS AND CORNEAL MELT
Epithelial removal and inadequate medication use may lead to infectious keratitis and corneal melting in patients treated with CXL. Irradiation parameters used for CXL with photoactivated riboflavin (ie, PACK-CXL) are similar to those used for conventional CXL. PACK-CXL, however, has been shown to kill more than 98% of pathogens, so why would infectious keratitis occur after conventional CXL? It is likely that mishandled open surfaces and an improper use of medications contribute to the occurrence of these responses, as shown in the following case.
Case report. A 36-year-old medical tourist presented with bilateral keratoconus. The patient underwent bilateral CXL in April 2012. Postoperatively, she wore contact lenses and applied nonsteroidal anti-inflammatory drug (NSAID) drops four times daily. In addition to their anti-inflammatory effect, NSAIDs also have an anesthetic effect on the cornea. Thus, when instructed to use NSAID drops only a few times per day, many patients may abuse the drops because they provide pain relief. The patient’s first follow-up visit occurred 4 days postoperatively, when it was discovered that an excessive application of NSAID drops had led to corneal melting through the induction of matrix metalloproteinases (Figure 5).
To prevent corneal melting, surgeons must respect the open surface. Whenever possible, NSAIDs should not be used postoperatively, and patients should be seen on a tight follow-up schedule.
TISSUE OXYGENATION
Although we have been performing CXL on patients since 1999, there are still many unknown factors, and it is possible that, when we encounter one of them, we may experience an unexpected response. In working both clinically and in the laboratory to answer some of these open questions, my colleagues and I believe we have identified a common denominator, which is oxygen.
So far, when talking about CXL and refining its techniques and technology, the focus has been on two main elements: UVA light and riboflavin. Once it was determined how to get enough riboflavin into the cornea, it was assumed we would have effective CXL. However, when surgeons attempted to redefine the method by accelerating the procedure and to make it more comfortable for patients by leaving the epithelium on, the quality of the results dropped.6,7
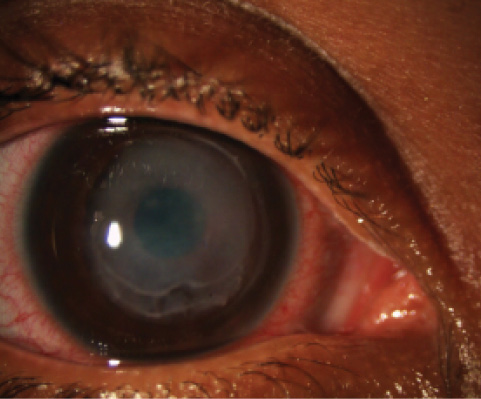
Figure 5. Corneal melt in a patient using excessive NSAID drops after CXL.
Why would the quality of results drop as we move away from the standard protocol? The answer might be the oxygen molecule. Oxygen is an essential component of CXL for keratoconus, as the cross-linking process implicates the generation of free radicals. We conducted an experiment in which CXL was performed in an oxygen-depleted, helium-filled atmosphere and found that no biomechanical change occurred. Thus, based on our observations, we infer that, when oxygen is removed from the equation, there is no response.
The standard protocol of 30 minutes at 3 mW/cm2 may enable a steady state of oxygen consumption and redelivery through passive consumption, as compared with high-intensity CXL. The transepithelial approach may face the same challenge, because the oxygen consumption of the epithelium is 10 times higher than stromal oxygen consumption. While many of the intricacies of CXL remain unknown, it may be best to adhere to the standard protocol rather than going to the edges of the technique.
CONCLUSION
Standard CXL and PACK-CXL seem to be safe procedures with relatively few unexpected and surprising results when the limits of the methods are respected. Increased experience and published data will serve to elucidate many of the unanswered questions associated with CXL and help us to determine which protocol is best for our patients.
1. Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41(1):41-46.
2. Hafezi F, Koller T, Vinciguerra P, Seiler T. Marked remodelling of the anterior corneal surface following collagen cross-linking with riboflavin and UVA. Br J Ophthalmol. 2011;95(8):1171-1172.
3. Koppen C, Gobin L, Tassignon MJ. The absorption characteristics of the human cornea in ultraviolet-a crosslinking. Eye Contact Lens. 2010;36(2):77-80.
4. Wollensak G, Spoerl E, Reber F, Seiler T. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye (Lond). 2004;18(7):718-722.
5. Richoz O, Tabibian D, Hammer A, et al. The effect of standard and high-fluence corneal cross-linking (CXL) on cornea and limbus. Invest Ophthalmol Vis Sci. 2014;55(9):5783-5787.
6. Hammer A, Richoz O, Arba Mosquera S, et al. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Invest Ophthalmol Vis Sci. 2014;55:2881-2884.
7. Caporossi A, Mazzotta C, Paradiso AL, et al. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39(8):1157-1163.
Farhad Hafezi, MD, PhD
• professor of ophthalmology, University of Geneva, Geneva, Switzerland
• chief medical officer, ELZA Institute, Dietikon/Zurich, Switzerland
• professor of ophthalmology, Department of Ophthalmology, University of Southern California, Los Angeles, California
• farhad@hafezi.ch
• financial interest: none acknowledged


