
We have been performing femtosecond laser cataract surgery in our practice since 2011, first with the LenSx Laser System (Alcon) and later with the Catalys Precision Laser System (Abbott Medical Optics).
Both laser systems have evolved in ways that have mitigated some initial weaknesses. The Catalys, for example, has become significantly faster, and the SoftFit interface for the LenSx has nearly eliminated problems with incomplete capsulotomies and skip tags due to applanation-related distortion.
Nearly 80% of the cataract procedures in our practice are now performed with the aid of a laser. Based on considerable experience with the technology, it is clear to me that the use of laser cataract surgery provides a number of benefits. In my opinion, the greatest benefits of the procedure are seen in the new information provided by the laser’s imaging capabilities, lens fragmentation, and the creation of uniform, precise capsulotomies.
IMAGING
Both the LenSx (Figure 1) and Catalys incorporate spectral-domain optical coherence tomography (OCT) imaging. They take snapshot images using OCT when assessing and setting the safety parameters. The Catalys also shows streaming OCT during the final approval of the safety fits.
Other available systems use various imaging technologies. The Victus (Bausch + Lomb) laser uses real-time streaming swept-source OCT (Figure 2), and the Lensar Laser System (Lensar) uses Scheimpflug-guided imaging. I would encourage anyone considering purchasing a laser cataract system to pay close attention to imaging capabilities, resolution, artifacts, and signal-to-noise ratio as part of his or her research into the devices.
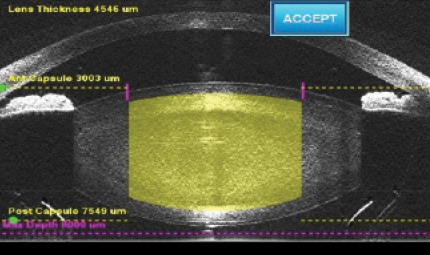
Figure 1. The LenSx Laser incorporates spectral-domain OCT imaging.
Figure 2. The Victus laser uses real-time, streaming swept-source OCT.
A significant advantage of the femtosecond laser—and one that was possibly not anticipated in the early days of laser cataract surgery—is that the incorporated OCT imaging gives the surgeon much more information about the anatomy of the eye, the anterior chamber, and the lens thickness. Although this information is obtained preoperatively with optical biometry, the ability to visualize the anatomy in real time during laser use allows the surgeon to anticipate needs prior to surgery that may improve outcomes. This truly makes me a better surgeon. Knowing how big the crystalline lens is in relation to the anterior chamber helps me prepare for surgery and may eventually allow me to more accurately predict the effective lens position (ELP).
For example, in a short eye with a shallow chamber, if the crystalline lens is of normal thickness, I would expect the IOL that replaces it to sit slightly forward and therefore have more power. If my lens power calculations suggest that a 26.00 D lens would give me a plano result and a 26.50 D lens would produce a -0.33 D result, I would thus choose the 26.00 D IOL. If, instead, I find a large, 5-mm-thick crystalline lens, I might make the opposite choice, because I would expect the lens to sit farther back in that capsule. Nomograms for crystalline lens thickness have not yet been quantified, but several surgeons are working on exactly that. There is tremendous potential to better understand and predict ELP based on the anatomic information obtained from the femtosecond laser.
Further, knowing the lens thickness and density also gives me a better idea of how far I can safely sculpt down, perhaps going deeper with a large lens and being more careful with a thin lens.
I particularly like the fact that safety zones are automated on the Catalys laser. The system uses sophisticated algorithms to process volumetric OCT image data, map the surfaces of the cornea and lens, and create safety zones, which I can then move or customize if necessary. Although the surgeon sees only cross-sections initially (Figure 3), the final approval screens are in real time, and the laser uses the full volumetric data for surface fits and treatment planning (Figure 4). This improves the accuracy of the safety zones and the quality of lens fragmentation.
With the Catalys, the surgeon sees both an axial and sagittal image, providing a more three-dimensional view of ocular structures. This is helpful in accounting for tilt or image artifacts. Being able to confirm something seen in the axial image by consulting the sagittal image increases my confidence in making a decision to redock to get rid of tilt, for example, or to move the safety zone for the posterior lens surface.
A few caveats can help to maximize the accuracy of OCT imaging:
• Insist on perfect docking, even if it takes a little more time.
• OCT imaging requires a clear cornea, so if there is arcus, corneal scarring, or increased pannus, you have to adjust treatment zones and the length of arcuate incisions based on corneal findings.
• OCT devices must be serviced, especially as surgical volume increases. If the image becomes grainy or pixilated, contact the laser manufacturer.
LENS FRAGMENTATION
Lens fragmentation is a real strength of the Catalys system. The surgeon has options to customize the number of laser passes and the treatment of the lens according to personal preference and nuclear density.
For cataracts up to grades 2 or 3, I segment the lens into six pieces. For grades 3 to 4+ or denser, I segment the lens into quadrants, but I also perform a fragmentation grid pattern within each quadrant. Many surgeons use fragmentation and segmentation in every case, it is important to be aware that the use of grid fragmentation can lead to loss of the red reflex. This can be disconcerting for the new laser cataract surgeon.
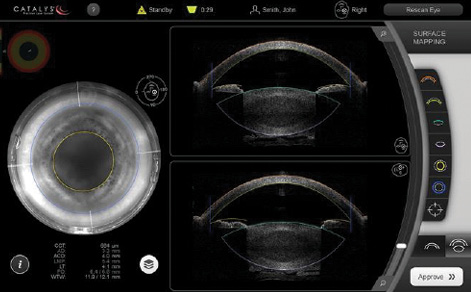
Figure 3. The Catalys laser shows the surgeon both axial and sagittal images, initially as cross-sections.
Figure 4. The Catalys final approval screens are in real time, with the laser using full volumetric data to generate surface fits and treatment planning.


