The ability to reproducibly automate a perfectly sized and circular capsulotomy has spurred much of the current clinical interest in laser cataract surgery. Compared to a manual continuous curvilinear capsulorhexis (CCC), the laser capsulotomy is highly reproducible and uniformly more circular, and it has a more precise diameter. These advantages come at a much higher capital and procedural cost, however, and they require a disruption of the normal surgical workflow, because the steps of laser cataract surgery cannot be performed within the usual operative sequence. Furthermore, differing national regulations may ban or restrict practices’ ability to balance bill patients for the additional costs associated with laser cataract surgery. Added to these drawbacks is concern over published reports of an increased rate of anterior capsular tears after laser capsulotomy.1,2 Scanning electron microscopy (SEM) of laser anterior capsular buttons has demonstrated a rougher edge compared to manual CCC specimens. SEM analysis has also revealed scattered aberrant laser shots that may be explained by microscopic eye movements during the laser capsulotomy step.1 These might be postulated to predispose focal areas of the anterior capsular rim to radial tears caused by subsequent surgical forces.
At a Glance
• Unlike the sequential circular path of a manual capsulorhexis or laser capsulotomy, the precision pulse capsulotomy technology mechanically and simultaneously cleaves all 360º of the apposed capsule without cauterizing it.
• The obvious potential advantage of the Zepto is its ability to reproducibly automate the capsulotomy step with a disposable instrument that is inserted in the conventional surgical sequence and is used in lieu of a capsulorhexis forceps.
• The Zepto can serve as an intraocular visual axis alignment device that allows the surgeon to center the capsulotomy on the patient-fixated coaxial sighted corneal light reflex.
• The results of extensive preclinical testing have been extremely promising.
New technology may confer the advantage of a precise, automated capsulotomy without the disadvantages of laser cataract surgery.
THE DEVICE
Mynosys has developed a novel capsulotomy method and technology called precision pulse capsulotomy (PPC) and trade named Zepto.3 A small console powers a disposable handpiece and nanoengineered capsulotomy tip to automatically and instantaneously create a perfectly circular capsulotomy of a precise predesigned diameter (Figure 1). The tip consists of a circular nitinol ring surrounded by a thin silicone suction cup (Figure 2). Nitinol is a superelastic shape memory alloy, which means that a ring of 5 or 5.5 mm in diameter can be compressed and deformed for insertion through a clear corneal incision but will re-assume its natural circular shape once it emerges inside the anterior chamber (AC). After the AC is filled with an ophthalmic viscosurgical device, a retractable metal push rod elongates the ring and silicone shell into a narrower profile that can be inserted through a 2.2-mm clear corneal incision. Once this push rod is retracted, the compressed tip resumes its circular shape within the AC.
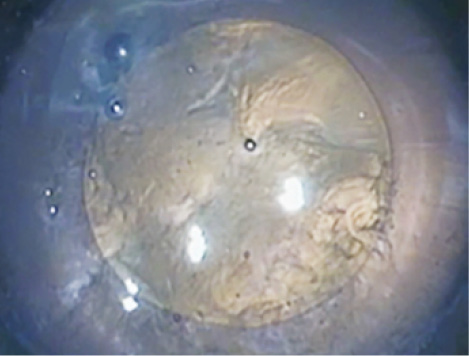
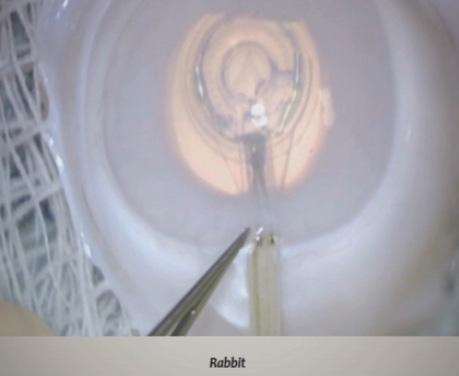
Figure 1. An example of a PPC in a human cadaver eye. The cornea has been removed to aid visualization.
The surgeon gently positions the ring and surrounding suction cup on the anterior capsular surface prior to applying a small amount of suction via the external console. Only slight suction is needed to appose the anterior capsule against the bottom edge of the nitinol ring, which has been precisely engineered at the micron scale to enable uniform cutting of the capsule. A rapid series of microsecond-long electrical pulses creates the capsulotomy. Phase transition of water molecules trapped between the capsule and nitinol edge causes the stretched capsular membrane to split circumferentially all at once. Unlike the sequential circular path of a manual capsulorhexis or laser capsulotomy, the PPC technology mechanically and simultaneously cleaves all 360º of the apposed capsule without cauterizing it (Figure 2). Collateral ocular tissue safety is achieved through two design features. First, the application of energy is extremely brief and confined to the microscopic edge of the nitinol ring. Second, during activation, the nitinol ring is completely covered by the silicone suction cup and further insulated by the surrounding ophthalmic viscosurgical device.
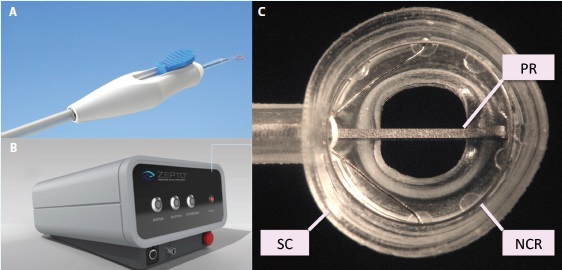
Figure 2. The Zepto consists of a disposable handpiece (A) attached to a control console (B) that provides power and suction for the capsulotomy. The handpiece terminates in a capsulotomy tip (C) consisting of a soft, clear silicone suction cup (SC) that houses a circular, collapsible, superelastic nitinol capsulotomy ring (NCR) to perform the capsulotomy. An extendable-retractable push rod (PR) helps to compress the capsulotomy tip for entry through a 2.2-mm corneal incision.
RESEARCH
The Zepto was developed through extensive testing in animal and human cadaver eyes. Miyake Apple view video imaging shows insignificant zonular traction during the PPC in both rabbit and human cadaver eyes. Preclinical performance and safety testing in live rabbits, including slit-lamp evaluation and histopathology, has been performed at the John A. Moran Eye Center in Salt Lake City in collaboration with Nick Mamalis, MD, and Liliana Werner, MD, PhD.3 These results showed no difference in postoperative inflammation, corneal edema, or endothelial cell loss when compared with fellow control eyes that received a manual CCC. There was no significant heat generation when measured by thermocouple experiments in live rabbits.
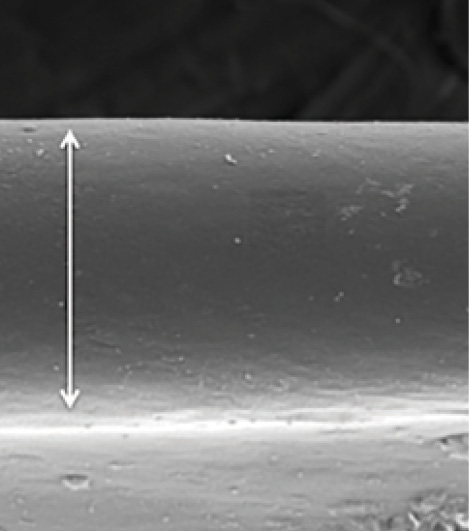
Figure 3. SEM shows that the PPC edge has a unique morphology characterized by an extremely smooth functional or working edge (arrow). In a detailed analysis, the Zepto not only created a perfectly round, tag-free opening in the capsule, but it also placed a microscopic upturning or eversion at the edge to present a small amount of the capsule’s defect-free underside for maximal edge integrity during surgery.
On SEM of human cadaver capsules, the PPC edge is smoother and freer of defects than the edge of a manual CCC (Figure 3). Vance Thompson, MD; John Berdahl, MD; Joel Solano, MD; and I have performed extensive testing of capsulotomy edge strength by comparing the PPC to both a manual CCC and laser capsulotomy in paired, fellow, human cadaver eyes.4 The PPC edge has consistently been stronger than either a manual CCC or laser capsulotomy (8/8 pairs for each comparison).
BENEFITS
The obvious potential advantage of the Zepto PPC is its ability to reproducibly automate the capsulotomy step with a disposable instrument that is inserted in the conventional surgical sequence and is used in lieu of a capsulorhexis forceps. Surgeons would undoubtedly welcome a method by which to ensure a perfectly sized and round capsulotomy without the workflow challenges and increased procedural time of laser cataract surgery. As with laser capsulotomy, popular indications would be complicated cases or eyes receiving premium IOLs. The lower cost should make PPC available to all patients regardless of their financial background and regional regulations of balance billing. Owing to the device’s efficiency, surgeons may opt to use the Zepto routinely rather than only in select cases, particularly if the PPC is faster and more consistent on average than a manual capsulorhexis. Although individual patients might still choose laser cataract surgery, surgeons could consider the Zepto for patients who cannot afford, do not want, or do not qualify for laser cataract surgery. I understand that the Zepto will cost approximately $100 and the control console $2,500. Given its operating principles of suction and electrical energy, the Zepto can also be easily integrated into phaco consoles.
Because it is an integrated step of conventional phacoemulsification, the PPC can be performed after the insertion of iris expansion devices for small pupils. The Zepto’s tip is also designed for insertion through a small pupil if necessary. Additional surgical steps such as capsular staining are not required. If the PPC edge is indeed stronger than the laser capsulotomy edge, the former may improve surgical safety by reducing the incidence of anterior and posterior capsular tears.
Finally, the transparent silicone suction cup has a central window that is designed to permit the patient to fixate on the microscope light filament during positioning of the device. Being able to center the capsulotomy on the visual axis would be advantageous for the implantation not only of multifocal IOLs but perhaps toric and even monofocal lenses as well.
CONCLUSION
In the metric scale, zepto is one million times smaller than femto. Both the small size of the instrument and the several-millisecond speed of capsulotomy creation inspired the name of the device. The Zepto received the CE Mark and is awaiting FDA 510(k) approval. The results of extensive preclinical testing have been extremely promising. Human clinical testing is the next step.
1. Abell RG, Davies PE, Phelan D, et al. Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery. Ophthalmology. 2014;121(1):17-24.
2. Chang JS, Chen IN, Chan WM, et al. Initial evaluation of a femtosecond laser system in cataract surgery. J Cataract Refract Surg. 2014;40(1):29-36.
3. Chang DF, Mamalis N, Werner L. Precision pulse capsulotomy—preclinical safety and performance of a new capsulotomy technology [published online ahead of print November 12, 2015]. Ophthalmology. doi: 10.1016/j.ophtha.2015.10.008.
4. Thompson VM, Berdahl JP, Solano JM, Chang DF. Comparison of manual, femtosecond laser, and precision pulse capsulotomy edge tear strength in paired human cadaver eyes. Ophthalmology. In press.
David F. Chang, MD
• clinical professor at the University of California, San Francisco
• (650) 948-9123; dceye@earthlink.net
• financial disclosure: has received compensation as a consultant to Abbott Medical Optics, Lensar, and Mynosys