All high-quality optical lenses are made with an aspheric design. That is true of telescopes, microscopes, eyeglasses, 20.00 D lenses for indirect ophthalmoscopy, and—for the past 15 years—IOLs.
Light passing through the center of a spherical lens—a lens designed as a section of a sphere—is focused at a different point than light passing through the periphery of the lens. The normal paraxial rays intersect the optical surface at a less acute angle, are refracted less strongly, and come together at a focal point farther from the back surface of the lens than the peripheral rays, which intersect the optical surface at a more acute angle and come together at a focal point closer to the back surface of the lens (this is an application of Snell’s law). Because all the light rays do not focus at a single point, the contrast of the image is reduced relative to the contrast of the object. Objects appear fuzzier, which makes them harder to detect and more difficult to recognize.
Reduced contrast is not the same as blur. The latter can be corrected by the right optical prescription (sphere and cylinder). Reduced contrast from a spherical lens can only be corrected by reducing the spherical aberration inherent in the lens. Spherical aberration is a symmetrical higher-order aberration that reduces image contrast, because all the light does not come to a single focus. In the pyramid of Zernike coefficients, spherical aberration is represented by the term Z(4,0).
As with all higher-order aberrations, spherical aberration is dependent on the aperture size (through a pinhole, spherical aberration disappears). Spherical aberration is, in fact, the variation of defocus with the radius of the aperture. Reducing spherical aberration enhances contrast. Objects become easier to see without spherical aberration—especially objects that have little contrast such as a white car on a snowy highway in the fog at dusk.1
When we are young, our eyes naturally have very little spherical aberration. The crystalline lens and cornea work together to maximize contrast; they actually balance each other to create an optimal visual image. The cornea maintains positive spherical aberration, whereas the lens has negative spherical aberration, thanks to the radial gradient in its refractive index. (The paraxial rays come to a focus closer to the back surface of the lens than the peripheral rays, because the index of refraction of the nucleus is greater than the index of refraction of the cortex.)
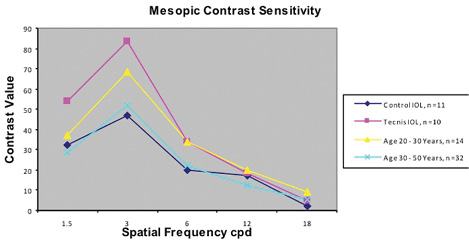
Figure 1. Patients implanted with an aspheric IOL demonstrated peak mesopic contrast sensitivity similar to that of phakic 20- to 30-year-olds and superior to that of both patients implanted with a spherical IOL and phakic 30- to 50-year-olds. (Reprinted with permission from Packer M, Fine IH, Hoffman RS. Functional vision, wavefront sensing, and cataract surgery. Int Ophthalmol Clin. 2003 spring;43(2):79-91.)
As we age, the crystalline lens starts to change. The spherical aberration of the normal lens increases throughout life, and as a result, the quality of the images we see decreases. (The spherical aberration of the cornea, on the other hand, remains relatively stable.) Even before we develop presbyopia, or cataracts, the quality of our lenses begins to decline. Past the age of 40, the crystalline lens is not doing us any favors!2
It makes sense that implanting an IOL more like the natural youthful lens, with negative spherical aberration, produces less spherical aberration and better contrast sensitivity overall. The Tecnis IOL (Abbott Medical Optics) was developed and rigorously tested in the first few years of this century (Figure 1).3 Its optical design incorporated a modified prolate anterior surface to create negative spherical aberration, like that of the youthful crystalline lens. Not only did it improve contrast sensitivity, but this IOL also significantly improved night-driving performance when compared to a spherical IOL. In an analysis conducted by the Potomac Institute, the impact of the Tecnis lens on highway safety was found to be greater than that of the center high-mounted brake light mandated on cars when Elizabeth Dole was the US Secretary of Transportation.4
The clinical outcomes demonstrated with the Tecnis IOL led the Centers for Medicare & Medicaid Services on January 26, 2006, to designate aspheric IOLs as a new class of New Technology IOLs.5 “Today’s announcement of coverage with additional payment for an innovative type of intraocular lens reflects Medicare’s attention to improved clinical benefits,” said Centers for Medicare & Medicaid Services Administrator Mark McClellan, MD, PhD. “For these lenses, there is clear evidence of improved functional vision and contrast acuity.”6
The concept of the aspheric IOL rapidly gained traction among ophthalmologists. Today, the vast majority of IOLs implanted in the United States are aspheric. In addition to monofocal IOLs, manufacturers have also designed toric, multifocal, and accommodating IOLs on aspheric platforms. Optimizing the performance of these implants, however, requires a thorough understanding of several key issues.
PUPILLARY SIZE
As mentioned earlier, spherical aberration depends on aperture size. With a 3-mm pupil, little difference can be found in the ocular wavefront between eyes implanted with aspheric or spherical IOLs, but the differences are pronounced with a 5-mm pupil.7 For this reason, greater dissimilarity may also be found when measuring mesopic contrast sensitivity or night-driving performance, because the pupil is naturally larger in dim light.
TILT AND DECENTRATION
Tilting or decentration relative to the optical axis degrades the performance of aspheric IOLs.8 Although the limits of tolerance are fairly forgiving,9 some caution is warranted when selecting patients for this technology (Figure 2). People with severe zonulopathy or intraoperative capsular complications that will affect the IOL’s stability may not be suitable candidates for negatively aspheric lenses. On the other hand, the performance of purely aspheric lenses such as the enVista IOL (Bausch + Lomb) is not reduced by decentration. The IOL most frequently recommended for placement in the sulcus, the AQ2010 (STAAR Surgical), is spherical.
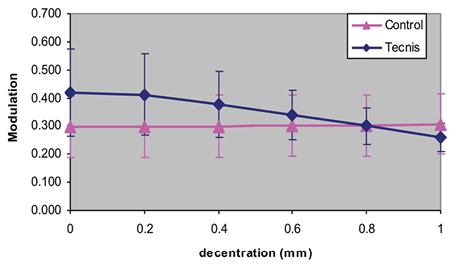
Figure 2. Decay of the modulation transfer function of a decentered aspheric IOL intersects the modulation transfer function of a spherical IOL at 0.8 mm.
DEPTH OF FOCUS
Better optical quality without spherical aberration sacrifices some depth of focus.10 One approach to this dilemma has been a compromise in IOL optical design that simultaneously maximizes both optical quality and depth of focus with a purely aspheric IOL (Z[4,0]=0). The enVista IOL follows this paradigm.
An interesting innovation involving depth of focus and spherical aberration is the programmed induction of negative spherical aberration to increase depth of focus after the implantation of the Light Adjustable Lens (Calhoun Vision; not available in the United States).11 The near vision obtained with this method is superior in eyes that have a larger magnitude of spherical aberration.11
CUSTOMIZING ASPHERICITY
In their review of aspheric IOLs, Montes-Mico et al noted that surgeons should “try to customize the asphericity depending on the patient’s corneal spherical aberration to obtain the optimum visual performance.”12 The Tecnis IOL was developed based on the mean corneal spherical aberration measured at the 6-mm optical zone in a cohort of European cataract patients, +0.27 μm. This implant was designed to complement the average cornea, leaving the eye with zero residual spherical aberration. The distribution of corneal spherical aberration in those populations that have been studied, however, is Gaussian. Among North American whites, Z(4,0) measures +0.274 ±0.089 μm.13 In the Japanese population, corneal spherical aberration has been measured at +0.203 ±0.100 μm.14
Given the range of aspheric IOLs available today, one might select a specific IOL to best achieve a given target for total spherical aberration in any particular eye.15 This approach might be particularly advisable for eyes with a history of keratorefractive surgery. For example, myopic LASIK generally results in a larger magnitude of positive spherical aberration than found in virgin eyes.
DEFOCUS AND ASTIGMATISM
Blur from residual uncorrected refractive error in pseudophakic patients can easily render moot the advantages of aspheric IOLs. To reap the benefits of increased contrast sensitivity from aspheric optics, sphere and cylinder should be reduced as much as possible and practical. Biometry and IOL power calculations as well as intraoperative aberrometry continue to play a crucial role in obtaining optimal outcomes, particularly for patients desiring postoperative spectacle independence.
CONCLUSION
The development and adoption of aspheric IOLs has advanced optical quality for pseudophakic patients and enhanced the concept of refractive cataract surgery. Providing youthful vision—in terms of both image quality and freedom from presbyopia—has become the goal of every refractive cataract surgeon. n
1. Piers PA, Manzanera S, Prieto PM, et al. Use of adaptive optics to determine the optimal ocular spherical aberration. J Cataract Refract Surg. 2007;33(10):1721-1726.
2. Packer M, Fine IH, Hoffman RS. Wavefront technology in cataract surgery. Curr Opin Ophthalmol. 2004;15(1):56-60.
3. Artal P. History of IOLs that correct spherical aberration. J Cataract Refract Surg. 2009;35(6):962-3; author reply 963-964.
4. McBride DK, Matson W. Assessing the significance of optically produced reduction in braking response time: possible impacts on automotive safety among the elderly. Arlington, VA:Potomac Institute for Policy Studies; April 1, 2003.
5. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Regulations-and-Guidance/Regulations-and-Policies/eRulemaking/downloads/CMS-3144-NC-1-11.pdf. Accessed February 20, 2015.
6. CMS announces approval of new technology intraocular lens. Centers for Medicare & Medicaid Services website. January 26, 2005. http://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2006-Press-Releases-Items/2006-01-26.html. Accessed February 20, 2015.
7. Kohnen T, Klaproth OK, Bühren J. Effect of intraocular lens asphericity on quality of vision after cataract removal: an intraindividual comparison. Ophthalmology. 2009;116(9):1697-1706.
8. Fujikado T, Saika M. Evaluation of actual retinal images produced by misaligned aspheric intraocular lenses in a model eye. Clin Ophthalmol. 2014;8:2415-2423.
9. Packer M. Tilt and decentration: toward a new definition of tolerance. EyeWorld. May 2005. http://bit.ly/1OaRWmh. Accessed February 20, 2015.
10. Marcos S, Barbero S, Jiménez-Alfaro I. Optical quality and depth-of-field of eyes implanted with spherical and aspheric intraocular lenses. J Refract Surg. 2005;21(3):223-235.
11. Villegas EA, Alcón E, Mirabet S, et al. Extended depth of focus with induced spherical aberration in light-adjustable intraocular lenses. Am J Ophthalmol. 2014;157(1):142-149.
12. Montés-Micó R, Ferrer-Blasco T, Cerviño A. Analysis of the possible benefits of aspheric intraocular lenses: review of the literature. J Cataract Refract Surg. 2009;35(1):172-181.
13. Beiko GH, Haigis W, Steinmueller A. Distribution of corneal spherical aberration in a comprehensive ophthalmology practice and whether keratometry can predict aberration values. J Cataract Refract Surg. 2007;33(5):848-858.
14. Shimozono M, Uemura A, Hirami Y, et al. Corneal spherical aberration of eyes with cataract in a Japanese population. J Refract Surg. 2010;26(6):457-459.
15. Packer M, Fine IH, Hoffman RS. Aspheric intraocular lens selection based on corneal wavefront. J Refract Surg. 2009;25(1):12-20.
Mark Packer, MD, CPI
• president, Mark Packer MD Consulting, Boulder, Colorado
• mark@markpackerconsulting.com
• financial disclosure: consultant to Advanced Vision Science, Alcon, Bausch + Lomb, Rayner Intraocular Lenses, and STAAR Surgical


