CASE PRESENTATION
—Case prepared by Lisa Brothers Arbisser, MD.

BROCK K. BAKEWELL, MD
First, I would refer the patient to a retina specialist for treatment of the flap tear in his right eye. After waiting 3 to 4 weeks to minimize the risk of retinal detachment, I would offer the patient phacoemulsification of the cataract in his right eye to rehabilitate his vision and enable him to drive. I would perform the procedure with a dispersive ophthalmic viscosurgical device (OVD) and a quick chopping technique.
I would then offer the patient the option of rehabilitating his left eye. If the cataract proved too hard for phacoemulsification, a planned extracapsular cataract extraction (ECCE) using a temporal approach would be my default procedure. I would create a 12-mm, 300-µm groove with a diamond micrometer blade at the most peripheral aspect of the temporal clear cornea. After injecting a dispersive OVD through a paracentesis incision, I would create a 2.5-mm entrance into the anterior chamber at the 3-o’clock position. I would place four iris hooks through sclerotomy incisions, 1.5 mm posterior to the limbus, to avoid tenting the iris. I would avoid using a Malyugin Ring (MicroSurgical Technology), because a large black cataract likely would not fit through the device for manual expression. After painting the anterior capsular surface with trypan blue dye under the OVD for better visualization, I would use a forceps to create a 7-mm capsulotomy.
Next, I would attempt phacoemulsification using a quick chopping technique. If the cataract were rock hard, I would convert to an ECCE, because squeezing the cataract through a small incision would damage more endothelial cells. Corneal decompensation may occur with any cataract extraction technique, so I would counsel the patient about the possible need for endothelial transplantation.

ASHA BALAKRISHNAN, MD
I would first address the right eye. Given the history of retinal detachment in the left eye, I would begin by referring the patient to a retina specialist for laser retinopexy around the torn flap and an evaluation for any other retinal pathology. After the patient’s clearance by the retina specialist, I would proceed with cataract extraction. I would pay special attention to protecting the endothelium through a soft-shell viscoelastic technique and frequent injection of an OVD during phacoemulsification. I would also attempt to perform as much phacoemulsification in the bag or at the iris plane as possible to minimize endothelial stress. Because this patient depends on his right eye, an IOL power targeted for distance would be best.
The visual acuity of the left eye is likely reduced secondary to the cataract. Given the low endothelial cell count and extremely dense cataract, traditional phacoemulsification is unlikely to succeed, and the cumulative energy used in the attempt could lead to corneal decompensation. With such a low endothelial cell count and a high probability of corneal decompensation, I believe combining manual small-incision ECCE with Descemet stripping endothelial keratoplasty would be the best approach. The choice of IOL may be limited, because the capsule may be so infused into such a mature cataract that it will be removed along with the lens. An IOL implanted in the bag or a three-piece IOL placed in the sulcus would be the most direct choices, but in the absence of a capsule and considering the diffuse iris transillumination defects, a scleral-fixated lens would be my choice.

WHAT I DID: LISA BROTHERS ARBISSER, MD
I counseled the patient about his increased risk of a retinal detachment in his right eye owing to the retinal tear, which required prophylactic laser retinopexy. I advised him that, despite the treatment and then waiting at least 6 weeks, cataract surgery would further increase his risk of a detachment. I recommended cataract surgery for the left eye first after immediate treatment of the torn flap in his right eye.
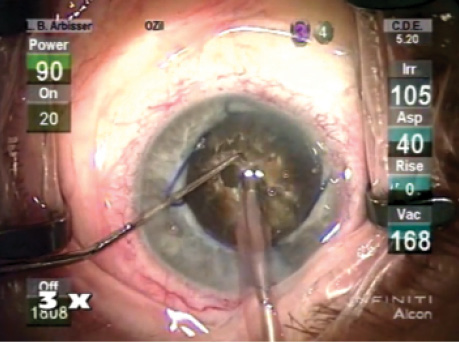
Figure. The eye at the start of surgery.
The patient understood his risk of requiring lamellar corneal surgery for corneal decompensation and the potential need for a capsular tension ring because of zonular abnormalities. I gave him a guarded prognosis for driving vision. I further explained that he might need more than the average postoperative treatment for inflammation. We decided to proceed with cataract surgery on his left eye first. He was able to fixate on a light, so topical anesthesia was adequate (Figure).
I started the patient on a topical nonsteroidal anti-inflammatory drug 1 week prior to surgery. I added scopolamine to my preoperative dilation routine. Based on the low cell count, I used BSS Plus and a DuoVisc soft shell (both products from Alcon), with an adequate dispersive OVD coating for the endothelium. I employed a Malyugin Ring after a mild two-point stretch of the iris. Next, I stained the capsule with trypan blue dye, because there was no red reflex for the capsulorhexis.
After establishing the mobility of the nucleus with multidirectional hydrodissection, I used the circumferential disassembly technique with cross-action vertical chop that I reported in 2004.1 With this technique, I make only a 0.5-mm paracentesis 90º away from the temporal clear corneal incision so as to reduce leakage, minimize fluid use, and control the intraocular environment. I use a Rosen splitter with a blunt tip as the chopper and a Kelman phaco tip with the sleeve retracted as far as possible while not allowing irrigation into the incision tunnel, which would risk chamber collapse. This approach facilitates vertical chop and provides a dipstick-like visual clue to confirm the tip’s safe depth in the nucleus when the tip is embedded to the edge of the sleeve. I prefer to enter the chamber on foot position zero by first filling the anterior chamber with a dispersive OVD. I find that this strategy prevents the patient from flinching and permits a controlled entry so that I can visualize Descemet membrane without damaging it.
I begin in foot position two to establish flow, thus avoiding wound burn and removing superficial cortex. On burst mode, with the panel-set longitudinal phaco power tailored to the density of the nucleus (80% ultrasound for brunescent lenses), I direct the tip into the lens so that it reaches just beyond half depth near the center. With vacuum engaged, I use the splitter to slice into the nucleus on the far side of the phaco tip. The two instruments are moved in opposite directions (cross action) to allow a fault line to form that, in a brunescent lens, will not reach the posterior plate but results in a superficial crack. Because the posterior plate of these lenses has rubbery interdigitated fibers, attempting to create two hemispheres with the first chop places unacceptable stress on the bag and leaves large pieces with unprotected capsule. Instead, my goal is to pry the two hemispheres apart slowly to about the middle of the depth of the nucleus only. No sculpting is required.
Repeatedly turning the lens and making small radial pie wedges permits the nucleus to be flayed open enough for me to engage the endonucleus, analogous to removing a clam from its shell. Although a mature lens cannot be hydrodelineated, this plane still exists, and it allows me to debulk the nucleus bit by bit in a circumferential manner from inside out while simultaneously creating my usual radial disassembly chops. I rotate the lens, usually many times, without placing stress on the zonules, and the outer shell of nucleofied epinucleus keeps the bag well expanded, thereby protecting the capsule, which has no cortical cushion. In addition, this step prevents fragile zonules from allowing the posterior capsule to become flaccid while I evacuate multiple sections of endonucleus in the safe zone within the continuous curvilinear capsulorhexis and deep in the chamber, away from the endothelium.
With burst mode, almost all of the ultrasound energy is used with the phaco tip occluded, either to “lollipop” and apply mechanical force or to assist aspiration in fragment removal. Very low cumulative dispersed energy is used, as in this case, which required less than 36 seconds. Virtually all of the ultrasound energy is delivered within the posterior chamber or at the iris plane—very sparing of the endothelium.
After uniformly debulking the endonucleus for 360º, I replace the dispersive OVD through the paracentesis if the viscoelastic has dissipated. I then lift the remaining thin “nucleofied” epinuclear shell into the anterior chamber and feed it into the phaco tip with the Rosen splitter. I step down the vacuum and flow rates to minimize surge during removal of the final fragments. At the same time, I carefully keep the second hand instrument below the phaco tip to protect the posterior capsule when actually applying ultrasound bursts.
Mature cataracts sequester cortex in the periphery. Systematic removal is necessary to leave a clean bag and minimize postoperative inflammation. Standard implantation of the IOL in the bag followed by removal of the Malyugin Ring and the OVD leaves a clean eye with intact incisions. Prophylactic hypotensive medication prevents a postoperative IOP spike.
In this case, although there was mild corneal edema on day 1, it had cleared completely by the 1-week visit. The patient enjoyed his best macular visual potential of 20/30, which allowed us to proceed with cataract surgery on the fellow eye in due time.
1. Arbisser LB. Clam shell circumferential disassembly for brunescent cataract. Video Journal of Cataract and Refractive Surgery. 2004;XXII(1).
Section Editor Lisa Brothers Arbisser, MD
• emeritus position at Eye Surgeons Associates, the Iowa and Illinois Quad Cities
• adjunct professor, John A. Moran Eye Center, University of Utah, Salt Lake City
• (563) 343-8896; drlisa@arbisser.com
• financial interest: none acknowledged
Section Editor Brandon D. Ayres, MD
• surgeon in the Cornea Service, Wills Eye Hospital, Philadelphia
Section Editor Audrey R. Talley Rostov, MD
• private practice with Northwest Eye Surgeons, Seattle medical advisory board, SightLife, Seattle
Brock K. Bakewell, MD • codirector, Fishkind and Bakewell Eye Care and Surgery Center, Tucson, Arizona
• (520) 293-6740; eyemanaz@aol.com
• financial interest: none acknowledged
Asha Balakrishnan, MD
• cataract, cornea, and refractive surgeon at Colvard-Kandavel Eye Center, Encino, California
• (818) 906-2929; sabalakrishnan.md@gmail.com; Twitter @AshaBalaMD