An aging population combined with increased patient expectations poses challenges for the high-volume cataract surgeon. I have found that optimal patient outcomes can be achieved when systematic and consistent methods are employed in the perioperative environment. These methods, although simple, help to ensure high-quality outcomes for the thousands of patients we care for every year.
TECHNICIAN LIAISONS BOOST QUALITY ASSURANCE
At our practice, we rely on several tools for quality assurance at the time of cataract surgery. Many years ago I started using my technician to assist in the preoperative area of the surgery center. Following preoperative admission by the nurse, the technician visits with each patient, verifies lens implant information with the patient, reviews the planned end result target, answers the patient's questions, and conveys pertinent information to the nursing staff and surgeon. This technician liaison is an invaluable asset in a busy cataract surgery center.
We use our electronic health record system to provide a report for the day of surgery. This report, generated directly from the electronic health record system, provides a list of all the surgical patients for the day. The report includes the operative eye, planned anesthesia, end result target, IOL selection, pertinent information from the lens calculation, and any special notes pertaining to the surgical plan. This information serves as a quick reference guide for all surgical staff, and the information is verified against the office notes and patient history and physical. In this way, any discrepancy can be caught and addressed before the patient is brought into the OR.
In our surgery centers, all patients undergo preoperative admission by a registered nurse followed by an interview with an anesthesiologist or certified registered nurse anesthetist. Most patients receive topical anesthesia for cataract surgery and intravenous sedation; retrobulbar or peribulbar blocks are reserved for specific cases. We use two ORs to maximize efficiency with room turnover. A team of registered nurses and technicians works in a collaborative manner in the OR to provide exceptional patient care and efficient room turnover.
SURGICAL METHODOLOGY
I also use a consistent methodology for my surgical technique, which is outlined here and demonstrated in my accompanying Eyetube video (bit.ly/weinstein0220). For standard cataract surgery, I prefer the stop-and-chop phaco technique, a term popularized by Paul S. Koch, MD.1 I always begin by creating two paracentesis incisions fashioned in the superior and inferior limbus with a 0.9-mm steel blade from Mani. I have been using Mani disposable blades for years, and I appreciate the consistency of the blades' sharpness and the quality of the blade. I inject a mixture of 1% preservative-free plain lidocaine with preservative-free phenylephrine intracamerally through one of the paracenteses (off-label use). This mixture provides analgesia, enhances mydriasis, and reduces the chance of intraoperative miosis.
My OVD of choice for most maneuvers is DuoVisc (Alcon), and I prefer to inject the dispersive Viscoat (Alcon) just before constructing the main wound because it helps to maintain the anterior chamber and protect the endothelium (Figure 1). To create the primary wound, I use a 2.2-mm steel keratome, again from Mani. I like to start my incision at the posterior limbus, beginning in the conjunctiva and extending approximately 2 mm into the cornea (Figure 2). This nearly square incision ensures strong wound integrity and has minimal impact on astigmatism.
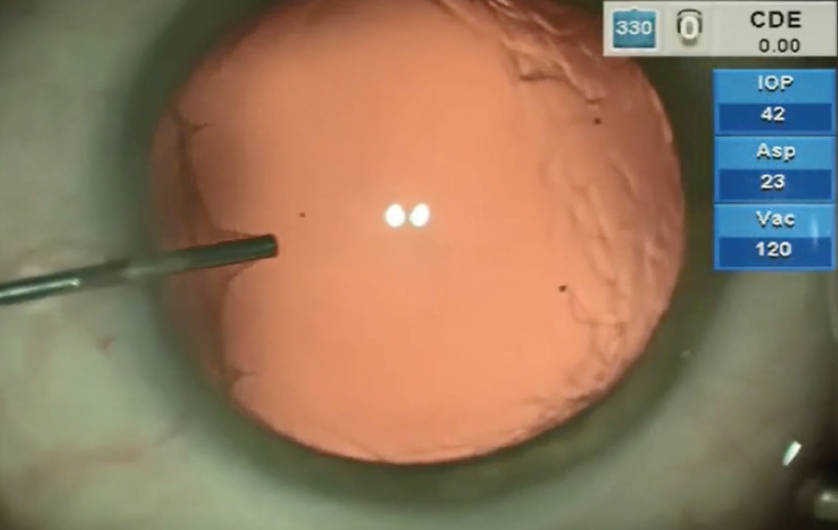
Figure 1. OVD is injected into the anterior chamber just prior to constructing the main incision.
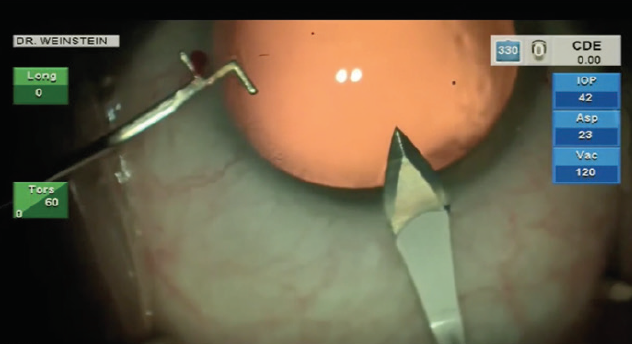
Figure 2. A 2.2-mm steel keratome is used to create the main incision at the posterior limbus. A sideport fixation hook keeps the eye stabilized.
Following incision creation, I make a slight cut-down of the conjunctiva to minimize chemosis during surgery. This can be done either with the keratome blade or with Vannas scissors. Because the wound is initially through the conjunctiva, a strong seal forms, which helps to prevent the introduction of bacteria. I have been performing this incision technique for many years and have not experienced a case of postoperative endophthalmitis in more than 15 years and more than 50,000 cases.
CAPSULORHEXIS, HYDRODISSECTION, AND PHACOEMULSIFICATION
For the capsulorhexis, I prefer to start with a bent 27-gauge cystotome needle. I find that this tool provides excellent feedback regarding zonular integrity, and it allows me to make adjustments to my capsulorhexis technique if I encounter weak zonules. I complete the 5.5-mm capsulorhexis with Gianetti capsulorhexis forceps (Katena).
In the presence of a very loose capsular bag, the surgeon must make a conscious effort to tear a large enough rhexis. Without this somewhat exaggerated effort, the rhexis will ultimately turn out smaller than planned, which can make the rest of the case much more challenging. A capsulorhexis of about 5.5 mm allows easier lens hydrodissection and removal of nucleus pieces, and it ensures good anterior capsule coverage of the lens implant.
Hydrodissection is achieved using a 26-gauge angled hydrodissection cannula. I never begin phacoemulsification unless I achieve adequate rotation of the nucleus with hydrodissection. I also like to hydrodelineate the nucleus when possible, but a very dense nucleus will not allow this.
After hydrodissection or hydrodelineation, I use the Intrepid Balanced Tip phaco needle with the Centurion Vision System (both by Alcon) for nucleus chopping and removal. I like to start by making a central groove in the nucleus, usually about 2 mm x 1.5 mm (Figure 3), applying footpedal control of continuous torsional phaco energy. I then spin the nucleus slightly counterclockwise in order to impale the distal nucleus with the phaco needle while placing my chopper in the previously created groove. A burst of longitudinal phaco is used to impale the nucleus, which is then held with high vacuum while a Sinskey hook is used to make the split.
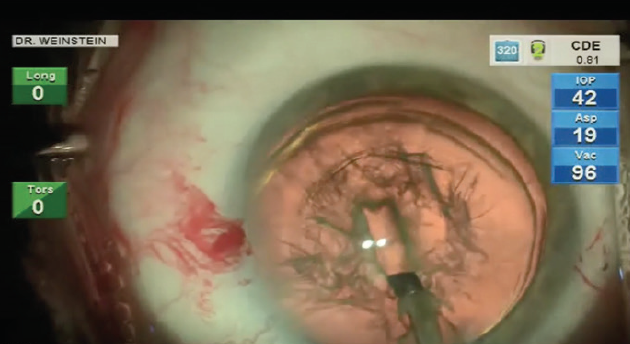
Figure 3. Stop-and-chop phaco is initiated with a short, deep groove.
I have used many nucleus choppers over the years but have found that the Sinskey hook is a simple and effective means to propagate a nice nucleus split. I continue to rotate the nucleus counterclockwise, performing the same maneuver of impaling and splitting with the Sinskey. The position of the Sinskey instrument is critical: It should be placed close enough to the phaco needle to facilitate a split in that plane, but not so close that it breaks the vacuum seal of the phaco.
For phaco chop settings, I like a fixed longitudinal burst, and I vary the on time depending on the nucleus density. I set a moderately high vacuum level to ensure a good grip of the nucleus when performing the chops.
After the nucleus is adequately chopped, I remove the pieces using a pulse phaco setting. I tend to mix torsional phaco with a small amount of longitudinal phaco. The Centurion’s Ozil Intelligent Phaco system (Alcon) allows the surgeon to program a specific amount of longitudinal phaco to kick in when a set vacuum limit is reached. For my personal technique, however, I employ a low amount of longitudinal phaco throughout nucleus removal, allowing efficient and safe nucleus removal without the necessity of programming the Intelligent Phaco. Along with my preferred phaco power settings, I use moderately high aspiration and vacuum. My goal in any nucleus removal is safety, so I tend to use conservative machine fluidics, and I adjust those based on the density of the cataract. For softer lenses, I often prechop the nucleus using an Akahoshi Combo Prechopper (Asico).
Following nucleus removal, I use a manual Simcoe I/A unit for cortex removal—the same tool that I have been using since my pre-phaco extracapsular cataract extraction days. Prior to using the I/A unit, I like to remove the subincisional cortex with a 23-gauge reverse cannula (Storz Ophthalmics) on a 3-cc syringe. The reverse cannula can be placed through either paracentesis to gently engage the subincisional cortex (Figure 4). I then place the Simcoe I/A through the main incision to provide a controlled method for cortex removal, which is particularly critical in the presence of zonulopathy. Attaching the Simcoe I/A to a 30-cc syringe allows the surgeon to manually control aspiration. After cortex removal, I use a Simcoe bulb/syringe (Storz Ophthalmics) to gently polish the posterior capsule.
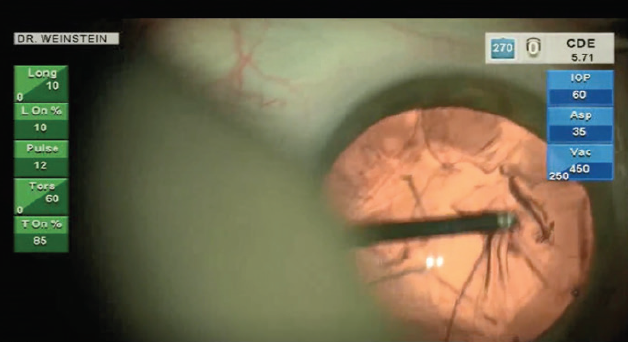
Figure 4. The anterior leaf of cortex is grasped in order to detach the subincisional cortex.
My typical monofocal IOL is the AcrySof IQ model SA60WF (Alcon), which has all the same properties as the SN60WF (Alcon) except that the SA model lacks the yellow chromophore. I inject the lens after I fill the anterior chamber with ProVisc (Alcon). Following IOL placement, the OVD is easily removed with the Simcoe I/A unit. Because this I/A device does not stretch the incision, wound closure occurs quickly at the conclusion of the case. I have not found it necessary to use stromal hydration of the main incision, as the wound is watertight at the end of surgery.
CONCLUSION
Many tools are available to the cataract surgeon to achieve successful surgical results for patients. Each surgeon should find a technique and appropriate instrumentation that allows him or her to deliver a consistent and safe surgical result.
I have found it helpful to use consistent personnel and technology whenever possible to assist in maintaining my high-volume cataract practice without sacrificing quality care. The busy surgeon can’t do everything alone, so it is important to surround oneself with a competent team, all working toward the same goal of achieving an excellent result for each patient.
1. Koch PS, Katzen LE. Stop and chop phacoemulsification. J Cataract Refract Surg. 1994;20(5):566-570.