
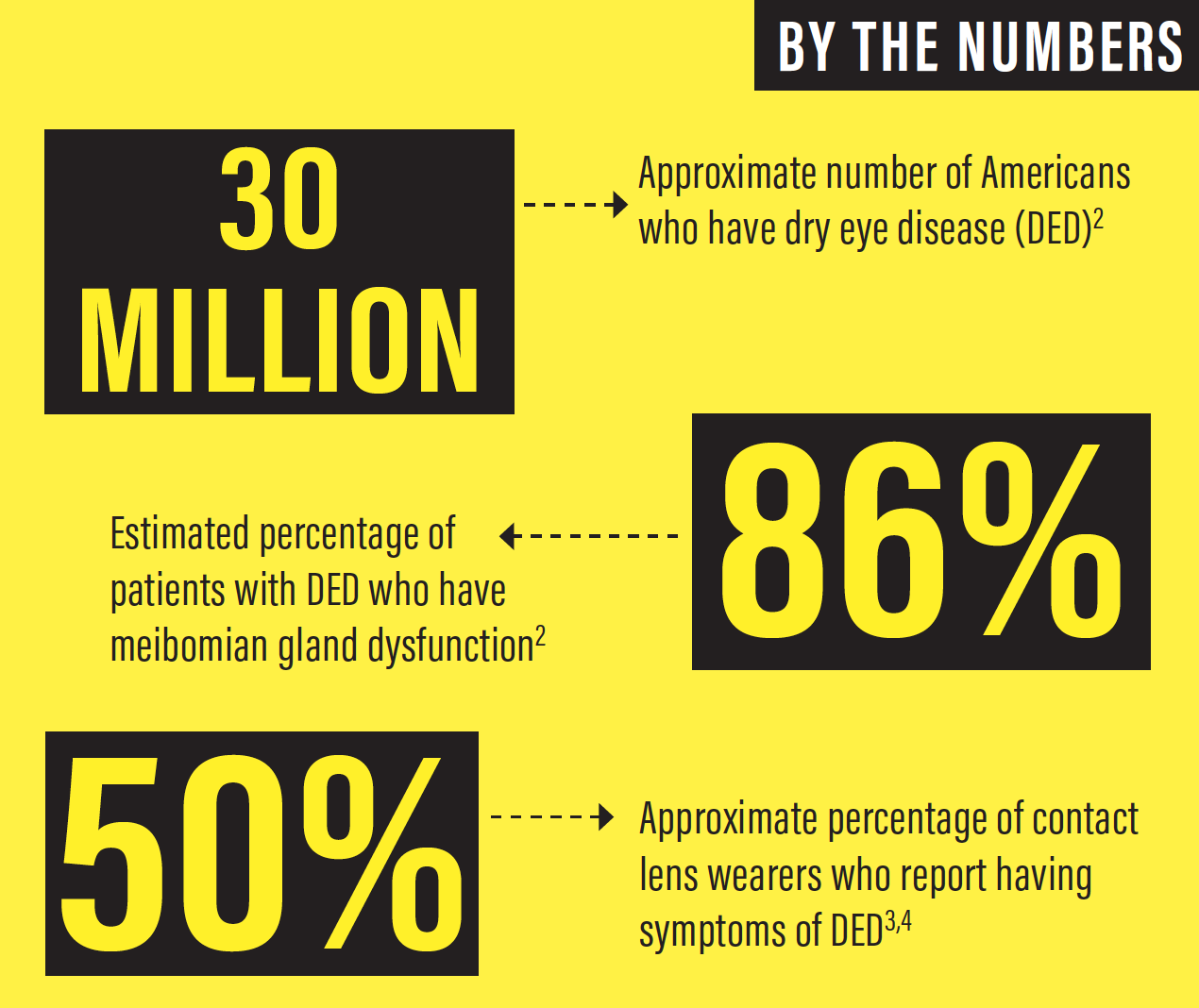
There is a high prevalence of ocular surface disease (OSD) in our population of prospective cataract and refractive surgery patients, and this means that we face a critical mass of people who need dedicated ocular surface rehabilitation before they undergo surgery (See By the Numbers below). Patients with systemic diseases such as diabetes, as well as those taking medications such as oral contraceptives and antihistamines, are all susceptible to dry eye disease (DED).
Approximately 5% to 10% of the patients I encounter have irreparable ocular surface damage that precludes them from being candidates for any form of refractive surgery, but the vast majority of people who have OSD can be considered for refractive surgery once their underlying disease is diagnosed and treated. Adequate time—ranging from 2 to 8 months—and a regimen dedicated to ensuring copious tear production and optimal meibomian gland function can transform almost any ocular surface to healthy tissue ready for refractive surgery.
PATIENT SCREENING
DED, a condition that was once considered a nuisance to treat, is now a booming specialty. Approximately 75% of patients in my practice get tested for DED. These patients are either referred for DED, are interested in cataract or refractive surgery, or have symptoms of DED. The high prevalence of DED suggests that screening and symptom assessment should be part of almost every eye exam.
This is particularly relevant for premium IOL patients, who tend to have high expectations regarding their postoperative vision. A compromised tear film can negatively affect the accuracy of biometry and therefore of subsequent IOL power calculations. A dry cornea makes it difficult to produce an accurate topographic map during evaluation for a toric IOL. Even with perfectly executed cataract surgery, postoperative DED negatively affects quality of vision. An otherwise successful procedure can be deemed a failure if the postoperative results do not meet the patient’s expectations.
SOLVING THE PUZZLE
When patients come in, I listen to their history and complaints and put the pieces of the puzzle together. Patients often arrive for a LASIK consultation explaining that they want refractive surgery because their contact lenses are increasingly uncomfortable to wear. Typically these patients need 2 to 4 months of ocular surface rehabilitation before they can be comfortable wearing contact lenses. At that point, I give them the option of continuing with their lenses or moving on to refractive surgery.
Other patients say they generally see well, but their eyes start to tear when they are driving, reading for hours, or watching television. All these activities reduce the blink rate, and a decreased blink rate promotes evaporation of the tear film and subsequent keratitis. The keratitis leads to symptoms of irritation, burning, and fluctuating vision. For these patients, I perform tear osmolarity testing. The numbers are highly reproducible, and the information this test yields is quite important.
I also use lisamine green vital dye to assess the conjunctival surface. The presence of staining indicates sloughing of the conjunctival epithelium secondary to a dessicated ocular surface. I also apply fluorescein dye to assess the corneal surface and see if the epithelial cells are damaged or dry (Figure 1).
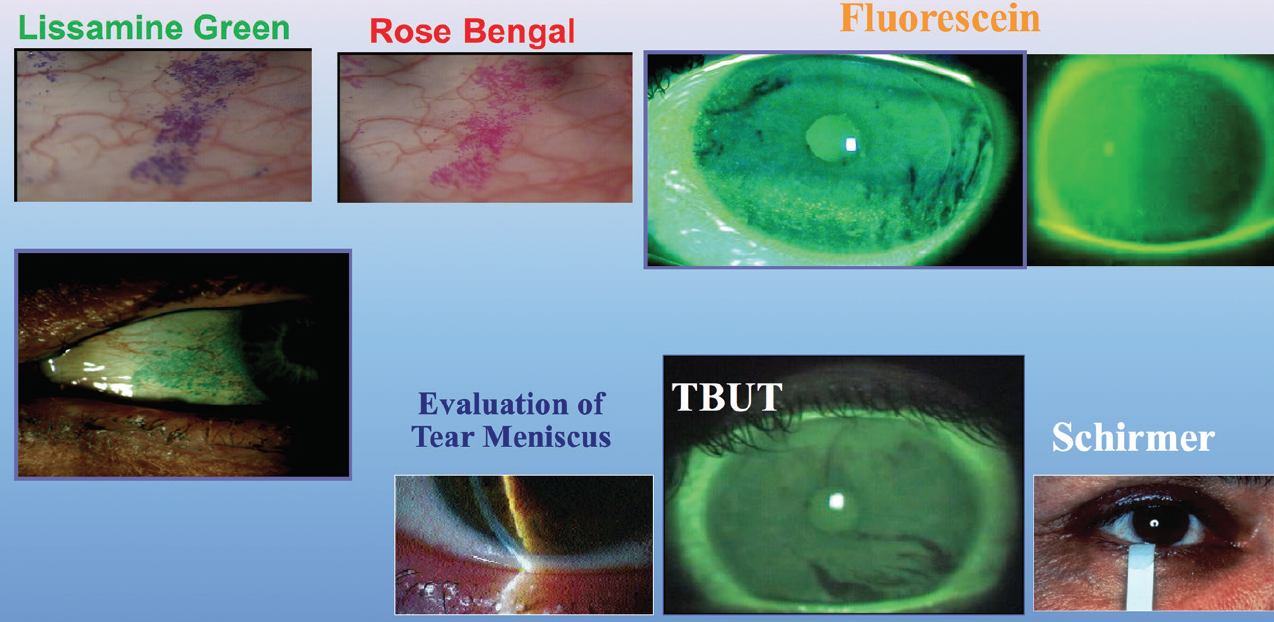
Figure 1. Common diagnostics for DED.
I perform Schirmer testing with anesthesia on most of my patients with DED to assess their baseline tear status. Patients who have less than 5 mm of wetting at 5 minutes are considered dry. I evaluate meibomian gland function with compression of the lid margins to view the quality and quantity of the meibum, and I perform meibography of the glands to view their structure. If the patient does not have a good oil layer, the tear film will evaporate more quickly.
After that, I look at the tear meniscus height (Figure 2) and the tear breakup time. I also perform manual keratometry on every patient. I am looking not only at the amount and axis of astigmatism, but also at the mires between blinks to ascertain whether the tear film is stable. I use all these data to develop a picture of the overall quality of the patient’s ocular surface.
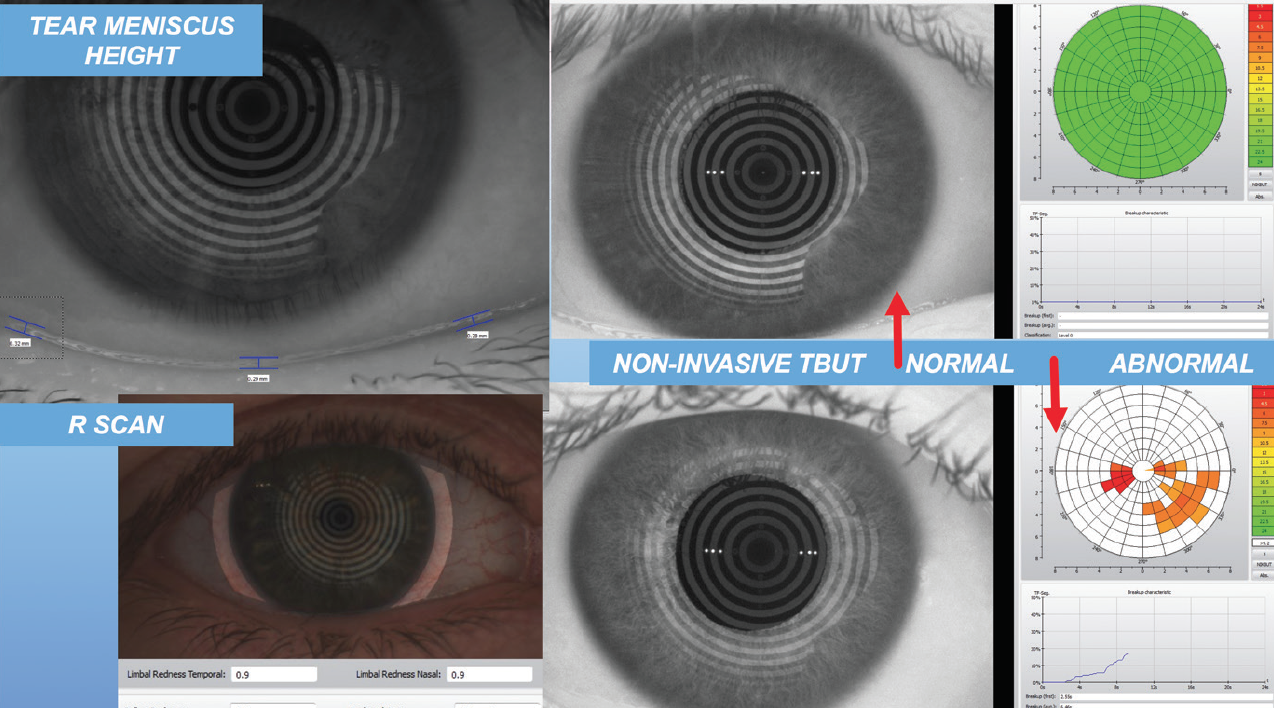
Figure 2. Tear meniscus height evaluation.
Next, I refract the patient and perform topography. If the topographic map shows a cornea that is not in perfect shape, I recommend to the patient that there should be no surgery until we rebuild the tear film over a period of typically 2 to 4 months—but even up to 1 year—because the cornea cannot heal sufficiently postoperatively without a healthy tear film.
It is not always easy for patients to accept this information. Sometimes they don’t want to dedicate the time to rehabilitating their ocular surface. Other times they can’t afford the out-of-pocket costs for the regimen of pharmaceuticals and supplements needed. The decision to comply with my recommended treatment is in their hands, but the decision to perform surgery is in mine, and I will not perform refractive surgery on someone with an insufficient tear film or otherwise compromised ocular surface.
RECIPE FOR REHAB
My recipe for ocular surface rehabilitation includes improving both the water and oil components of the tear film. I prescribe use of preservative-free artificial tears several times a day and then check back after about 1 week to see if the patient is more comfortable and if there is any improvement. If not, I move on to stimulation of the patient’s own tears with cyclosporine ophthalmic emulsion 0.05% (»Restasis; Allergan). If there is significant inflammation of the ocular surface, I add a low-dose topical steroid, loteprednol etabonate ophthalmic suspension 0.5% (»Lotemax; Bausch + Lomb) twice a day, and lifitegrast ophthalmic solution 5% (»Xiidra; Shire).
Next, I consider whether the oil secretion component of the eye is sufficient. To decrease inflammation and improve oil secretion, I prescribe 1,000 mg of omega-3-acid ethyl esters (Lovaza; GlaxoSmithKline) twice daily or another pure omega-3 fatty acid supplement (Omega Plus; Thorne Research) twice daily plus a hypochlorous acid spray (Avenova; NovaBay) twice daily. If necessary, I prescribe an oral antibiotic, not for the antibiotic activity but for the antiinflammatory activity. I like the tetracycline family, either doxycycline 100 mg twice daily or minocycline 100 mg twice daily. If the patient cannot tolerate these medications, then I substitute azithromycin, one tablet daily for 6 days, to be repeated every month.
I ask patients to return in 3 to 4 weeks to gauge their improvement and decide if they are ready for surgery or need to continue the rehabilitation. I err on the side of continuing treatment because I know they will get dryer as a result of the refractive surgery, and naturally I want them in tip-top shape before booking their procedure.
Ditch disappointing results
The majority of people who have refractive surgery go on to develop some degree of DED postoperatively.1 In my practice, all prospective patients watch a video explaining that, if they do not have dry eyes when they arrive seeking refractive surgery, they will develop dry eyes postoperatively, and the condition may last 3 months to 1 year.
Ultimately, the ocular surface rules the day. You can do everything right as far as your surgical technique, your IOL choice, or your LASIK algorithm, but if the tear film is compromised, your results will be disappointing.
1. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806.
2. Nichols KK, Foulks GN, Bron AJ, et al. The International Workshop on Meibomian Gland Dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929.
3. Dumbleton K, Caffery B, Dogru M, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on epidemiology. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS20-36.
4. Doughty MJ, Fonn D, Richter D, et al. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997;74(8):624-631.




