
Intracameral (IC) antibiotics have become essential in cataract surgery, particularly for the prevention of postoperative endophthalmitis (Figure), a rare but sight-threatening complication. This article explores the evolution of IC antibiotic use, its current application in practice, data on its effectiveness, its operational impact in high-volume settings, and potential future developments.

EVOLUTION AND ADOPTION OF IC ANTIBIOTICS
Previously, systemic antibiotics were the primary method for preventing postoperative infection. Studies, however, have shown that administering antibiotics directly into the anterior chamber at the end of cataract surgery more effectively reduces the incidence of postoperative endophthalmitis.
Cefuroxime was one of the first IC antibiotics to be widely used. Its popularity grew following a 2007 ESCRS trial that demonstrated a fivefold reduction in endophthalmitis rates with IC cefuroxime.1,2 Since then, antibiotics such as moxifloxacin and vancomycin have been introduced. IC antibiotics are now used routinely in many parts of the world, but the choice of agent varies based on factors such as availability, cost, and local microbial flora. Moxifloxacin is used predominantly in India, whereas vancomycin is more common in Australia and the United States.3
Despite the widespread adoption of IC antibiotics, controversy persists regarding their necessity, particularly given modern aseptic surgical techniques and the overall low incidence of endophthalmitis.3-10
EFFICACY OF IC ANTIBIOTICS
Key Clinical Trials and Findings
Numerous studies have documented the efficacy of IC antibiotics in preventing postoperative complications such as endophthalmitis (Table). In addition to the aforementioned landmark ESCRS trial,1,2 a noncontrolled retrospective observational study conducted by Montan et al in Sweden showed that the endophthalmitis rate dropped from 0.26% to 0.065% with the use of IC cefuroxime.24
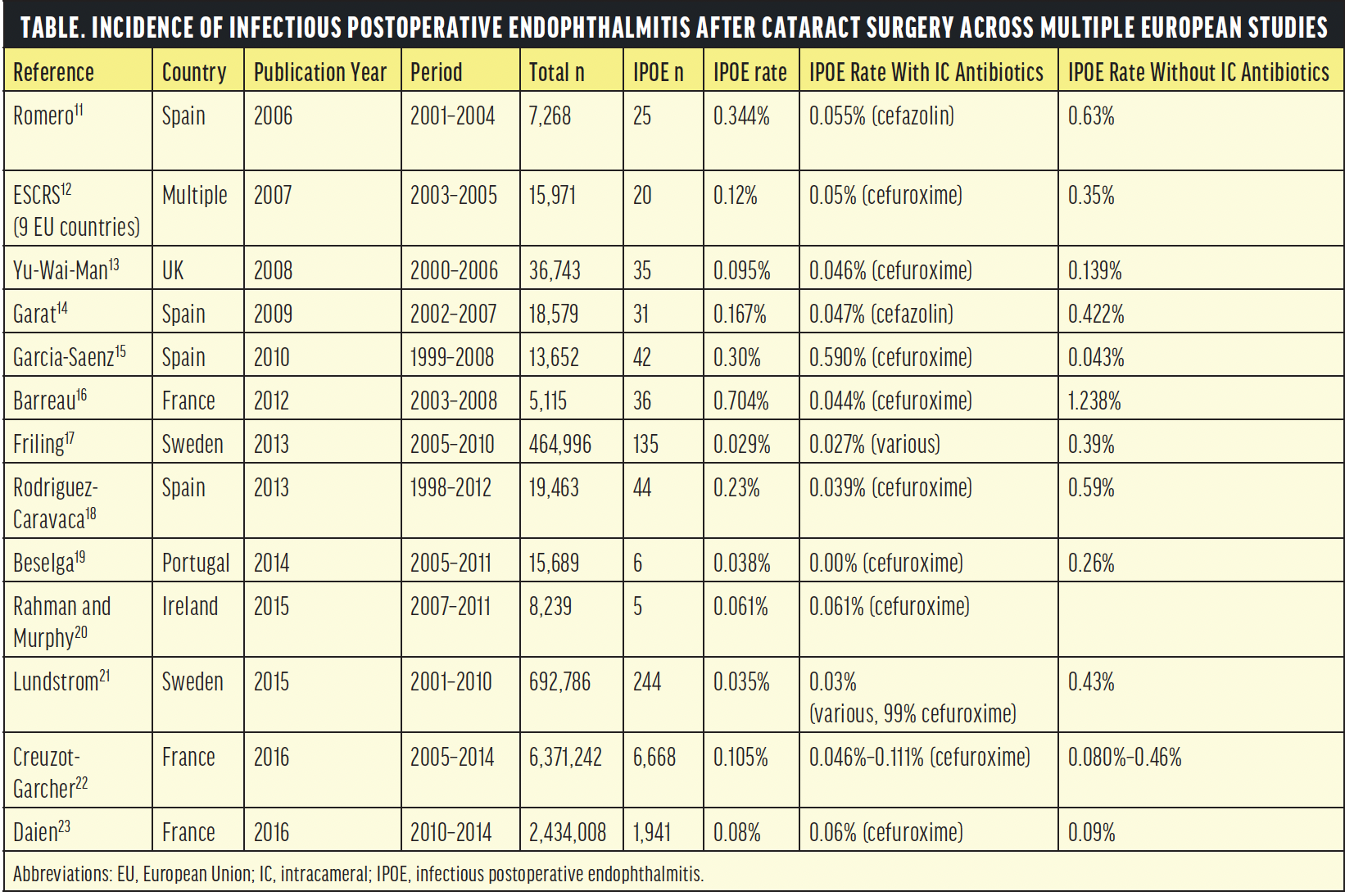
Other retrospective studies and meta-analyses have reinforced these findings. IC antibiotics have significantly lowered infection rates across various settings and populations.3-10 In the United Kingdom, Spain, France, and South Africa, endophthalmitis rates have been consistently lower in cohorts treated with IC antibiotics compared to those not receiving these drugs.3-10
Risks Associated With IC Antibiotics
Adverse events after the IC administration of antibiotics are rare. They include toxic anterior segment syndrome—particularly with improper dosing or compounding errors—and hemorrhagic occlusive retinal vasculitis (HORV) after vancomycin use.25,26
DRUG CHOICE
Again, the choice of prophylactic IC antibiotic varies globally, but the agents most commonly used are cefuroxime, moxifloxacin, and vancomycin.
Cefuroxime
The ESCRS Study provided substantial evidence supporting the use of IC cefuroxime.1,2
Moxifloxacin
Moxifloxacin is a fourth-generation fluoroquinolone that exhibits potent, concentration-dependent activity against gram-positive and gram-negative bacteria as well as atypical organisms. Increasing fluoroquinolone resistance among coagulase-negative staphylococci, however, is a concern.
One randomized controlled trial analyzed the use of moxifloxacin, but the study involved a small number of surgeries.27 Several retrospective studies have reported a low relative risk of endophthalmitis in patients treated with IC moxifloxacin compared to those who did not receive IC antibiotic prophylaxis. Large retrospective studies have shown a benefit in patients receiving IC moxifloxacin regardless of the type of cataract surgery performed. IC moxifloxacin is typically administered without dilution or at a dose of 500 mg. In India, moxifloxacin hydrochloride is widely used for IC antibiotic prophylaxis.3,10
Vancomycin
IC vancomycin has been widely used by cataract surgeons worldwide. Large retrospective studies have reported a significant reduction in the incidence of endophthalmitis with IC vancomycin. In 2014, however, severe cases of bilateral ischemic retinal vasculitis following IC vancomycin were documented, and cases of HORV associated with IC vancomycin have since been reported.25
HORV is characterized by painless vision loss and specific ophthalmic findings after an otherwise uneventful postoperative period.26 The HORV Task Force has documented cases of HORV following IC vancomycin and highlighted the challenges of diagnosing and managing these cases.
OPERATIONAL CHALLENGES AND BENEFITS IN HIGH-VOLUME SETTINGS
In high-volume surgical centers, the use of IC antibiotics presents both operational challenges and benefits.
The preparation and administration of these antibiotics must be meticulously managed to ensure patient safety and adherence to protocols. For example, preparing IC antibiotics such as cefuroxime requires strict aseptic conditions and adherence to compounding guidelines to prevent contamination and dosage errors. Dedicated pharmacy support and rigorous training for surgical staff are necessary. The need for single-use syringes and the potential for antibiotic shortages can strain resources.
The use of IC antibiotics can have a positive impact on patient throughput. The agents’ effectiveness in preventing infection reduces the need for additional postoperative care and readmissions due to endophthalmitis. The drugs’ efficiency not only enhances patient outcomes but can also improve overall workflow and the capacity of high-volume surgical centers.
Incorporating IC antibiotics into standard practice helps mitigate the risk of postoperative infection, which can have severe consequences for patients and legal implications for health care providers. The potential for adverse reactions, however, necessitates careful patient selection and monitoring.
POTENTIAL DEVELOPMENTS IN THE USE OF IC ANTIBIOTICS
New antibiotics are being developed that offer a broader spectrum of activity and have lower resistance potential. Additionally, advances in drug delivery systems, including sustained-release formulations, may enhance both the efficacy and safety of IC antibiotics.
Ongoing research into the genetic and microbial factors associated with endophthalmitis may pave the way for more personalized prophylactic strategies. For example, optimizing antibiotic selection based on a patient’s specific microbial flora and resistance profiles could improve clinical outcomes.
Another potential advance is the integration of IC antibiotics with other prophylactic measures, such as enhanced perioperative antiseptic protocols and advanced surgical techniques. A holistic approach combining these strategies could further reduce infection rates and enhance patient safety.
Greater collaboration and consensus-building among international ophthalmology societies could help standardize IC antibiotic practices globally. Such efforts could ensure that patients worldwide benefit from the latest evidence-based strategies for the prevention of endophthalmitis.
ADVANCING IC ANTIBIOTICS IN CATARACT SURGERY
The introduction of IC antibiotics has reduced the incidence of endophthalmitis after cataract surgery. Although controversies and challenges persist, the evidence strongly supports the efficacy of IC antibiotics in preventing postoperative infection. Ongoing research and innovation are expected to yield even more effective and safer prophylactic strategies, further enhancing patient outcomes and operational efficiency, particularly in high-volume surgical settings.
1. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
2. Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions 2013. ESCRS. Accessed August 12, 2024. https://retinabg.com/wp-content/uploads/2023/09/english_2018_updated.pdf
3. Grzybowski A, Brona P, Zeman L, Stewart MW. Commonly used intracameral antibiotics for endophthalmitis prophylaxis: a literature review. Surv Ophthalmol. 2021;66(1):98-108.
4. Grzybowski A, Turczynowska M. More antisepsis, less antibiotics whenever possible. Asia Pac J Ophthalmol (Phila). 2018;7(2):72-75.
5. Grzybowski A, Koerner JC, George MJ. Postoperative endophthalmitis after cataract surgery: a worldwide review of etiology, incidence and the most studied prophylaxis measures. Expert Rev Ophthalmol. 2019;14(4-5):247-257.
6. Villegas VM, Schwartz SG, Grzybowski A, Relhan N, Flynn Jr HW. Endophthalmitis prophylaxis: different practices from around the world. In: Das T, ed. Endophthalmitis. A Guide to Diagnosis and Management. Springer; 2018:345-356.
7. Grzybowski A, Turczynowska M. Endophthalmitis, toxic anterior segment syndrome, and vitritis. In: Narang P, Trattler W, eds. Optimizing Suboptimal Results Following Cataract Surgery: Refractive and Non-Refractive Management. Thieme; 2019;141-150.
8. Grzybowski A, Turczynowska M. Prophylaxis and treatment of endophthalmitis. In: Cataract Surgery. Springer; 2021;191-199.
9. Flynn HW Jr, Batra NR, Schwartz SG, Grzybowski A. Endophthalmitis in Clinical Practice. Springer; 2018.
10. Das T, Joseph J, Simunovic MP, et al. Consensus and controversies in the science of endophthalmitis management: basic research and clinical perspectives. Prog Retin Eye Res. 2023;97:101218.
11. Romero P, Méndez I, Salvat M, Fernández J, Almena M. Intracameral cefazolin as prophylaxis against endophthalmitis in cataract surgery. J Cataract Refract Surg. 2006;32(3):438-441.
12. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
13. Yu-Wai-Man P, Morgan SJ, Hildreth AJ, Steel DH, Allen D. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34(3):447-451.
14. Garat M, Moser CL, Martín-Baranera M, Alonso-Tarrés C, Alvarez-Rubio L. Prophylactic intracameral cefazolin after cataract surgery: endophthalmitis risk reduction and safety results in a 6-year study. J Cataract Refract Surg. 2009;35(4):637-642.
15. García-Sáenz MC, Arias-Puente A, Rodríguez-Caravaca G, Bañuelos JB. Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery ten-year comparative study. J Cataract Refract Surg. 2010;36(2):203-207.
16. Barreau G, Mounier M, Marin B, Adenis JP, Robert PY. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg. 2012;38(8):1370-1375.
17. Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39(1):15-21.
18. Rodríguez-Caravaca G, García-Sáenz MC, Villar-Del-Campo MC, Andrés-Alba Y, Arias-Puente A. Incidence of endophthalmitis and impact of prophylaxis with cefuroxime on cataract surgery. J Cataract Refract Surg. 2013;39(9):1399-1403.
19. Beselga D, Campos A, Castro M, et al. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5-year study. Eur J Ophthalmol. 2014;24(4):516-519.
20. Rahman N, Murphy CC. Impact of intracameral cefuroxime on the incidence of postoperative endophthalmitis following cataract surgery in Ireland. Ir J Med Sci. 2015;184(2):395-398.
21. Lundström M, Friling E, Montan P. Risk factors for endophthalmitis after cataract surgery: predictors for causative organisms and visual outcomes. J Cataract Refract Surg. 2015;41(11):2410-2416.
22. Creuzot-Garcher C, Benzenine E, Mariet AS, et al. Incidence of acute postoperative endophthalmitis after cataract surgery: a nationwide study in France from 2005 to 2014. Ophthalmology. 2016;123(7):1414-1420.
23. Daien V, Papinaud L, Gillies MC, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA Ophthalmol. 2016;134(7):810-816.
24. Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28(6):977-981.
25. Nicholson LB, Kim BT, Jardón J, et al. Severe bilateral ischemic retinal vasculitis following cataract surgery. Ophthalmic Surg Lasers Imaging Retina. 2014;45(4):338-342.
26. Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology. 2017;124(5):583-595.
27. Melega MV, Alves M, Cavalcanti Lira RP, et al. Safety and efficacy of intracameral moxifloxacin for prevention of post-cataract endophthalmitis: Randomized controlled clinical trial. J Cataract Refract Surg. 2019;45(3):343-350.




