Introduction
In April, a panel of surgeons with early experience using the Technolas TENEO 317 Excimer Laser Platform (Bausch + Lomb) convened to discuss the laser’s precise engineering, intentional efficiency, and unparalleled experience and outcomes. TENEO, approved by the FDA in November 2023, is designed for surgeons who are focused on outstanding patient outcomes and want a streamlined way to achieve them. It is the first excimer laser platform to receive FDA approval in nearly 2 decades.
Deepinder K. Dhaliwal, MD, L.Ac: The goal of our discussion is to elucidate the technology of the TENEO Excimer Laser System recently introduced by Bausch + Lomb. It’s been 17 years since we’ve had a new excimer laser, so I’m excited to delve into the details of the technology. Dr. Waring, you were the first surgeon in the United States to own a TENEO system. There are so many other excimer lasers on the market. What sets this one apart?
George O. Waring, MD, FACS: We participated as an investigator for the US FDA clinical trial for myopia and myopic astigmatism. Our hands-on experience with this laser was positive. The graphical user interface touchscreen is intuitive and allows customization of patient data screens; the user experience and outcomes are outstanding; and we realized efficiencies in workflow. When you put those together in a clinical trial, you get a sense that we’re onto something good. Our involvement in the clinical trial helped us make a pretty easy decision to purchase the TENEO. We introduced the technology to the United States in February 2024 with the acquisition of the first commercial TENEO and the first commercial treatments in the United States, and it has been a wonderful move for our practice.
PRECISE ENGINEERING
Dr. Dhaliwal: Dr. Tyson, you were the second surgeon in the United States to purchase a TENEO. Why the TENEO?
Farrell “Toby” Tyson, MD, FACS: I primarily perform cataract surgery. Although laser vision correction is not the focus of my practice, it still behooves me to have the best technologies available. For my practice, I wanted a machine that meets the following criteria: 1) reliable, 2) safe, 3) quick, 4) easy to use, and 5) has a small footprint. The last criterion is important to me because I don’t use the laser 7 days a week. Every square foot dedicated to a laser is space that could be used as another lane or product to generate revenue. Not only is the TENEO compact, taking up only 6.8 ft2—the smallest excimer laser available in the United States—it is also a user-friendly technology. My staff members are cross-trained, meaning they may be working in the OR one day and the clinic the next. The more convoluted a piece of equipment is, the harder it becomes for them to use. I want it to be simple to operate, and TENEO is just that. My staff no longer dreads firing up the excimer laser on refractive surgery procedure days. The three-step flow (select patient; choose and confirm treatment; and treat) works well for them, and we don’t have to work through nomograms because the software treats the manifest refraction and does not require a nomogram. This eliminates several preoperative steps, streamlining surgical planning. It’s nice being able to input the refraction and allow the machine to automatically do the treatment.

Karl G. Stonecipher, MD: I echo Dr. Tyson’s sentiments. My staff loves TENEO. When they are happy, life is much easier, and our surgery days go much quicker. I’ve had access to excimer lasers since about 1986, when laser refractive surgery was in its infancy. The Summit Apex (Summit Technology), VISX (Johnson & Johnson Vision), and WaveLight (now Alcon) lasers were ahead of their time, and now the TENEO technology is such a great advancement in modern refractive surgery.
The speed of the laser, for me, is a big deal. As early as the 1990s, we showed that speed made a difference in surgical outcomes.1 The slower the procedure time, the larger the risk for complications, including desiccation. Corneal relaxation time can also be an issue. Another thing I love about TENEO is the eye tracker, which works at roughly three times the repetition rate. It’s a pretty phenomenal laser.
Dr. Tyson: A fast eye tracker doesn’t matter unless it’s able to make an adjustment before the next spot, which the TENEO is capable of doing. As a cataract surgeon who sees a lot of post-refractive surgery patients with decentered ablations, the TENEO is a game-changer. We also saw really low rates of contrast sensitivity issues.
Dr. Waring: It is impressive. These are some of the best results we’ve seen to date.
Dr. Stonecipher: And surgery is just so streamlined now. The TENEO is easy to use for our staff and surgeons, and it is comfortable for the patient, who doesn’t have any trouble getting into position under the laser. I also appreciate that it has been engineered for the comfort and safety of both surgeon and patient.
Dr. Tyson: Some of us get a new iPhone every year. How long has it been since we’ve had a new excimer laser? Some surgeons have only ever used and known one laser system. Now, Bausch + Lomb is bringing a whole new world to them.
INTENTIONAL EFFICIENCY
Dr. Dhaliwal: Dr. Waring, can you share some insights into the laser’s design?
Dr. Waring: It has been quite a while since there has been new excimer laser technology, and TENEO has moved the needle. The clinical data from the FDA trial supports the outcomes, so not only is the TENEO’s ease of use and reduction in potential input error significant, but also the efficiencies and the outcomes are some of the best we have seen with excimer laser technology to be reported to date.
When one considers efficiencies in technology, the first word that comes to mind is intuitive. If it does the thinking for you, that’s when you have an ‘aha’ moment and know you are onto something special. That has been our experience with TENEO. The user interface is quite extraordinary. You plug in the manifest refraction, do your check, and perform the treatment.
Dr. Dhaliwal: With every other excimer laser technology I’ve used, a critical piece of the puzzle was the nomogram, which was based on your specific laser. Are you saying there is no nomogram needed for this laser?
Dr. Waring: That’s right—there is no nomogram required. The software is designed to treat the manifest refraction, and that is one of the most significant contributions that this technology brings to our profession.
Dr. Stonecipher: This is crucial. Residents, fellows, and new and seasoned refractive surgeons alike can struggle with their nomograms. TENEO has essentially eliminated that issue. Additionally, like Dr. Waring said, this streamlines our workflow.
Another factor worth mentioning is that most other systems require safety checks of the laser between patients, but TENEO doesn’t. When we’re talking about throughput, saving 30 seconds to 2 minutes may add up to 30 minutes per day. We do safety checks, but less often. That’s important.
Dr. Tyson: The laser’s integration and the design are second to none. For instance, the patient bed is designed to increase comfort for any patient type. With other lasers, I struggled to get barrel-chested people under the laser. TENEO allows you to customize the bed excursion in the swing because of its small footprint. This gives you maximum flexibility to treat more patients, and patients report that it’s not as scary for them. For doctors, the oculars provide multiple magnifications, and the slit beam is wonderful. All of these intricate details make TENEO a total package, not a bolt-on system.
Dr. Waring: Dr. Tyson talked about square-footage efficiencies. At half the size of some of the other lasers in the United States, TENEO creates an open area feel, so the experience for the patient is much more natural and comfortable. This matters because we want to create a positive experience for them. Vision correction is one of the most significant things we can do for our patients, and we want the experience to mirror that. We also want the experience to be superb for our staff and for ourselves.
UNPARALLELED OUTCOMES AND EXPERIENCE
Dr. Dhaliwal: It’s easy to appreciate the TENEO’s small size, fast treatment time, and intuitive design, but what we need to dive into are the refractive outcomes. Dr. Waring, are the results with this laser equivalent or better than results with other systems? Are patients going to embrace this technology?
Dr. Waring: As an investigator in the US FDA clinical trial for myopia and myopic astigmatism, the design of the excimer laser piqued our interest to what the laser could do in a real-life setting. The results of the clinical trial set the stage for realizing how the laser improves outcomes.
The US FDA study was a prospective, multicenter, open-label, nonrandomized, single-arm trial with 10 sites across the United States. All patients had either myopia of -1.00 to -10.00 D or myopic astigmatism up to -3.00 D. The trial was designed with no nomogram or physician adjustment made, so it was truly treating off the manifest refraction.
When we looked at postoperative uncorrected distance visual acuity (UCDVA) relative to preoperative best corrected distance visual acuity (BCDVA), 99.7% and 97.8% of patients were 20/40 or better and 20/25 or better for UCDVA, respectively (Figure 1). More than half of patients were 20/16 or better, and I think that’s what we’ve come to expect with modern excimer profiles and ablation patterns.

Figure 1. Comparison of postoperative cumulative UDVA and preoperative CDVA.
The changes in lines of vision and BCDVA were quite significant. In fact, about 30% and 4% of patients gained 1 or 2 lines relative to their preoperative status, respectively (Figure 2).
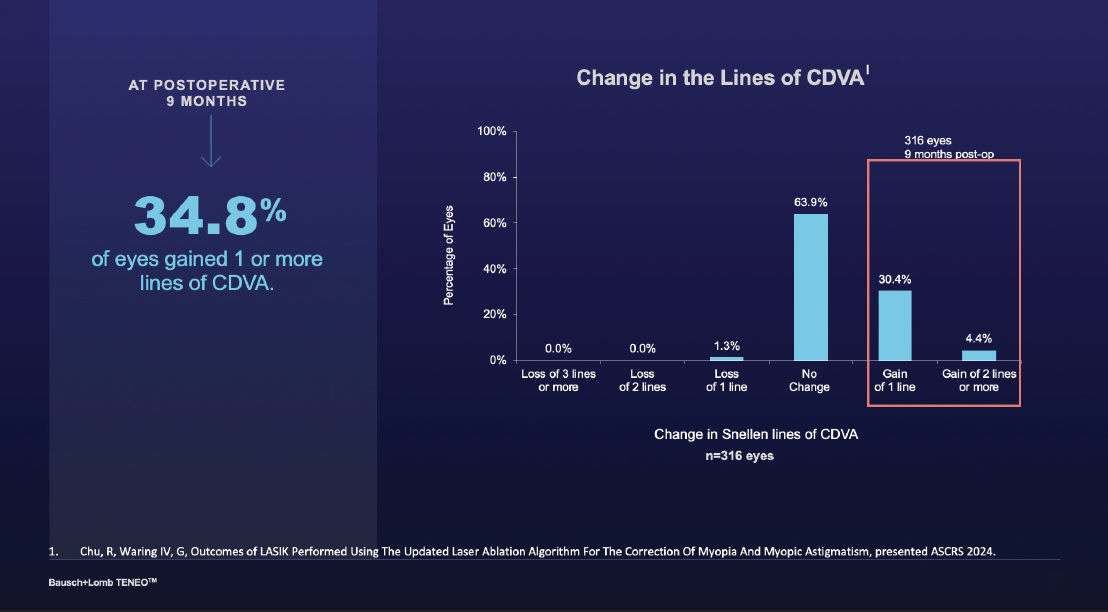
Figure 2. Change in the lines of CDVA from baseline to 9 months postoperative.
Dr. Stonecipher: To add to that point, nobody lost vision. I think not losing vision is huge in terms of 2 lines or more.
Dr. Waring: When we consider that there was no nomogram or physician adjustment, the real ‘aha’ moment for me was achieving a manifest refraction spherical equivalent (MRSE) of -0.04 D at 9 months.
Dr. Stonecipher: And if you look at the standard deviation, it was just one click on the phoropter.
Dr. Dhaliwal: Was there any regression?
Dr. Waring: No. The MRSE was maintained at our end point of 9 months. We were within ±0.50 D of the refractive target in 90.2% of patients (Figure 3).

Figure 3. Mean MRSE between 1 and 9 months postoperative.
Dr. Dhaliwal: Quality of vision is critically important for patients and surgeons. Dr. Stonecipher, what have you found in terms of these outcomes?
Dr. Stonecipher: Patient-reported outcomes after LASIK for myopia and myopic astigmatism showed significant improvements in specific areas like glare and halos, vision in dim light, and night driving (Figure 4). Postoperatively, there were fewer starbursts, glare, and halos than preoperatively. And I think that’s a big win, because patients with myopia don’t see well to begin with due to their long eyes. Night driving, both corrected and uncorrected, is a common struggle for these patients. I think the improvement in night vision is one of the top-tier results in terms of patient satisfaction (Figure 5).

Figure 4. Incidence of visual phenomenon at 9 months postoperative.

Figure 5. Patient-reported outcomes at 9 months postoperative.
Dr. Dhaliwal: That’s huge. During a preoperative consultation, I always tell patients, ‘You’re going to have more glare and halos. Your night driving might be more challenging initially.’ To see this kind of shift in this outcome is remarkable.
Dr. Stonecipher: The study data were collected unilaterally. Out in the real world, however, people use both eyes. From that vantage point, patients are performing spectacularly in their environments, whenever that may be, at 5 am or 9 pm.
Dr. Dhaliwal: Were patients happy with the results?
Dr. Stonecipher: Only fewer than 33% of patients were satisfied with their vision preoperatively, whereas 98% were satisfied after laser vision correction with this platform. This was statistically significant.2
Dr. Dhaliwal: Dr. Waring, how did contrast sensitivity compare pre- and postoperatively?
Dr. Waring: In addition to such positive subjective results, objectively, we saw patients’ contrast sensitivity improve. This is another unique moment for excimer refractive surgery. Historically, there has been an initial drop in contrast sensitivity after excimer laser treatment, but now we have a statistically significant objective improvement in contrast sensitivity from preoperatively to postoperatively. The US FDA clinical trial confirmed that we can improve quality of vision, not only subjectively, but also quantitatively as well with contrast sensitivity function.
Dr. Tyson: As a cataract surgeon seeing this data, I’m thinking that, if all things are equal between different platforms, then an excimer is an excimer is an excimer. We know that is not true, but why is this system so special? In my view, it’s being able to treat that eye efficiently with no wasted steps. The treatment plan is the treatment you are applying to the cornea; and it’s not changing as the eye is changing because it’s so fast. All these small enhancements help drive this type of outcome. To me, this is a big step forward. In addition to the positive clinical trial results, the fact that TENEO has been used extensively overseas made me comfortable being an early adopter of the technology.
Dr. Dhaliwal: Another important point about the improvement in contrast sensitivity is that perhaps these patients can be good candidates for advanced-technology IOLs in the future. I’ve always been a little reticent to put a multifocal IOL in patients who had previous refractive surgery, because I didn’t want to decrease their contrast sensitivity even more. This concern may not apply to patients who have had laser vision correction with the TENEO.
Dr. Waring: There are other aspects to this, too. To Dr. Stonecipher’s point, the laser’s repetition rate is 500 Hz, however the TENEO has the fastest treatment time in the United States. Why? First, it treats 1.00 D of refractive error in 1.2 seconds. It’s Proscan beam profile is a unique wavefront-optimized profile with radial ablation efficiencies and a truncated Gaussian beam that allows for more efficient treatments. It also has the fastest eye tracker in a commercially available US excimer laser. We surgeons can see the difference these advantages make when we look at the eye immediately postoperatively. The flap is clear and with minimal to no edema. I believe that this improves outcomes, speed of visual recovery, and in my experience, this difference also creates a ‘wow’ factor for patients.

Dr. Stonecipher: One other thing we haven’t yet talked about is that TENEO is diagnostic-agnostic. As businesspeople, we don’t want to invest in more diagnostic technologies beyond what we already have. And with TENEO, there is nothing new to buy; the laser is an addition to our office, but it’s producing phenomenal results in patients in an easy-button fashion. I believe that this factor improves outcomes.
Another thing that surprised me was that TENEO incorporates the Z axis into its eye tracker. This ensures the laser is delivering energy where it’s supposed to, and I think that also aids in producing better vision.
EXCEPTIONAL EXPERIENCE, RICH PARTNERSHIP
Dr. Dhaliwal: What I am hearing is that patients love their treatment experience and the staff loves it, too, because TENEO is easy to navigate. What about the surgeon’s experience? After a day of surgery, sometimes my neck hurts, and I’m tired. All of you have performed treatments using the laser platform. Tell us about your experience.
Dr. Waring: We love what we do, and we have access to intuitive and exciting technology that’s also fun to use. But, the surgeon’s experience really matters. The intuitiveness of the TENEO lightens my day, because I don’t have to do the mental gymnastics with a nomogram or physician adjustment.
Dr. Stonecipher: Talking about another kind of adjustment, this is one of the first platforms where you don’t have to wrap yourself around the laser. Body positioning is easy, because surgeons can adjust the laser for their comfort, whether they’re 6’6” or 5’6”.
Dr. Tyson: Even the joystick on the laser’s articulating arm can be set to where you want it; the oculars can be flipped, extended out, and pulled back; and the controls and main power screen (which is large!) are in the perfect position for me to see them. Now that I’m getting a little bit presbyopic, it’s nice to have larger fonts that I can read. Furthermore, the joystick can be moved up, down, left, and right. When I was in ophthalmic training, I was urged to get myself comfortable before I started a case. I didn’t see the value in it then, when I didn’t have long OR days. Now, 20 years later, I know how crucial it is to make myself comfortable, because I’m going to be in that position for a long time throughout the day. The patient is very comfortable, and if they’re comfortable, it’s a lot easier to do the treatment.
Dr. Dhaliwal: Partnership with industry is so important, especially when you’re learning a new technology. In your opinion, Dr. Stonecipher, how has Bausch + Lomb been as a partner?
Dr. Stonecipher: For us, the company has been awesome. Bausch + Lomb representatives have come to our practice several times to see how we use the laser and help us improve outcomes. Staff turnover is such an issue for all of us, and someone from Bausch + Lomb is always a phone call away if we need to train another staff member.
Dr. Tyson: My experience is similar. I was impressed by how accommodating Bausch + Lomb representatives were to me as a cataract surgeon. LASIK is something that I need to have access to, but it’s not a primary driver in my practice. They understood that, and they figured out how to fit the laser into my practice and make the system work in my setup and workflow. Having access to a laser that is straightforward, efficacious, and, frankly, makes financial sense for the practice and the patient is key.
Dr. Waring: Bausch + Lomb had the vision to invest in excimer laser vision technology, and ultimately the company has moved the needle to the next generation of refractive surgery.
Dr. Dhaliwal: Having a new excimer laser is revolutionary. I’m excited about all the things we’ve discussed. The results are phenomenal, especially the improvement in contrast sensitivity. You don’t need a nomogram, and you treat off the manifest refraction. It’s just very straightforward. I think the TENEO is groundbreaking. What excites each of you about the future?
Dr. Stonecipher: Refractive surgeons in the United States stand on the shoulders of international giants, like Sheraz M. Daya, MD, FACP, FACS, FRCS(Ed), FRCOphth, and Robert Edward Ang, MD, and others overseas who have access to technologies before we do. I’m hoping that we will be able to bring other treatments to this laser system. I think that it’s an exciting future with this laser platform.
Dr. Tyson: With excimer laser surgery, we’re exiting the excuses generation and entering the performance generation. Now, we’re talking about the positives and the experience that we can bring to the patient. We’re seeing the same thing with IOLs, and it’s making my practice much more fun, because now I’m providing patients better vision with lenses like Aspire™ and IC-8® Apthera™ (both from Bausch + Lomb). The enVista® Monofocal (Bausch + Lomb) also has outstanding results.
Dr. Stonecipher: One of the best things this laser platform brings is ease of use. One reason a lot of ophthalmologists don’t use premium IOLs is that they don’t want to deal with unhappy patients. IOL exchange is a big deal, and no one wants to go back to the OR. But in most cases, patients just need a little bit of a touch-up on the surface, and I think that if surgeons have a platform that makes it easier to perform a laser vision correction enhancement, it might just increase the number of premium IOLs they implant.
Dr. Waring: TENEO is an enabling technology—it’s allowing us to look forward to broadening indications for laser vision correction and treating more patients in ways we were not able to do before. Outside the United States, our colleagues have access to wavefront-guided treatments and are looking at topography-guided treatments with the TENEO. In the future, we may be able to do this in the United States to incrementally change the game. We are moving into an era where we can deliver a level of care and outcomes that were not achievable in the past.

CLOSING THOUGHTS
Dr. Dhaliwal: Before TENEO, we hadn’t seen an excimer laser come to market in 17 years. Now, with this development of precise engineering and intentional efficiency, it seems we can provide patients with unparalleled experience and outcomes. Dr. Tyson, would you like to offer any final pearls or thoughts?
Dr. Tyson: We’re seeing a resurgence in the laser vision correction market with a new generation of potential patients and renewed interest. The nice thing about having a new technology is that we can explain where we’ve come from and what we have now. There are no more excuses with TENEO, only high-performance benefits. In terms of cataract surgery patients who need a touchup, I can very easily place the patient under the laser and know it’s a pleasant experience for them. The treatment time, 1.2 seconds per 1.00 D, is so quick that the surgery is over before they even knew it began. To me, it really is being able to bundle that whole experience for patients that makes this laser so special.
Dr. Dhaliwal: Thank you all for sharing your pearls and really ‘telling it like it is’ in terms of your experience with the TENEO excimer laser. It’s exciting to have an amazing technology to explore further.
TEN.0058.USA.24
1. Stonecipher, K.G., Kezirian G.K., Stonecipher, K.G. LASIK for Mixed Astigmatism Using the ALLEGRETTO Wave: 3- and 6-Month Results with the 200-Hz and 400-Hz Platforms J Refract Surg 26(10):819-823,2010.
2. Stonecipher, K, Endl, E, Patient-Reported Outcomes Following Lasik, Performed Using A Novel Excimer Laser For The Correction Of Myopia And Myopic Astigmatism, presented at ASCRS 2024.
Important Safety Information
Indications for Use
The Technolas Teneo 317 Model 2 is indicated for laser-assisted in situ keratomileusis (LASIK) in: (1) Patients for the reduction or elimination of myopic astigmatism up to -10.00 D MRSE, with sphere between -1.00 D and cylinder between 0.00 and -3.00 D; (2) Patients who are 22 years of age or older; (3) Patients must have a stable refraction in the last 12 months, as documented by previous clinical recordings, i.e., the spherical and cylindrical portions of the manifest distance refraction have not progressed at a rate of more than 0.50 D per year prior to the baseline examination in the eye(s) to be treated.
Warning
Danger of injury due to failure to observe the patient selection criteria! Failure to observe the contraindications and potential adverse effects may result in serious permanent patient injury. The usage of the laser system is limited to a specific field of applications. Observe the contraindications and potential adverse effects listed in the User Manual before selecting a patient and starting any treatment.
Contraindications
Contraindications of the Technolas Teneo 317 Model 2 include patients: (1) with any type of active connective tissue disease or autoimmune disease; (2) with signs of keratoconus, abnormal corneal topography, and degenerations of the structure of the cornea (including but not limited to pellucid marginal degeneration); (3) with significant dry eyes (severe Dry Eye Syndrome). If patients have severely dry eyes, LASIK may increase the dryness. This may or may not go away. Severe eye dryness may delay healing of the flap or interfere with the surface of the eye after surgery. It may result in poor vision after LASIK; (4) for whom the combination of their baseline corneal thickness and the planned operative parameters for the LASIK procedure would result in less than 250µ of residual corneal thickness from corneal endothelium; (5) with uncontrolled diabetes; (6) with uncontrolled glaucoma; (7) with active eye infections or active inflammation: (8) with recent herpes eye infection or problems resulting from past infections; (9) with known sensitivity to medications used for standard LASIK surgery.
Potential Risks and Side Effects
(1) Miscreated flap; (2) Subconjunctival hemorrhage or bleeding; (3) Wrinkles in flap that may require a flap lift; (4) Corneal erosion/abrasion, epithelia defect; (5) Elevated IOP; (6) Debris or foreign body under flap; (7) Epithelial ingrowth under flap; (8) Debilitating visual symptoms, especially at night; (9) Decreased or fluctuating visual acuity; (10) Decreased ability to see in low-light conditions; (11) Light sensitivity; (12) Dry Eye syndrome; (13) Inadequate treatment result; (14) Regression; (15) Corneal damage; (16) Posterior vitreous detachment or retinal detachment, floaters or vascular accidents; (17) Foreign body sensation or pain (initial postoperative days); also, potentially including chronic eye pain that is resistant to therapy referred to as neuropathic pain; (19) Infection/inflammation; (20) CTK (Central Toxic Keratopathy); (21) Medication intolerance; (22) Ptosis; (23) Cataract; (24) Ocular penetration; (25) Potential risk of psychological harm.
This is not all you need to know. Please see the User Manual for a complete list of safety information, including a full list of contraindications, warnings, precautions and risks.
Caution: Federal (U.S.) law restricts this device to sale, by or on the order of a physician.







