Fifty Years of Evolution
James A. Davison, MD, FACS

Phacoemulsification has changed a lot since 2001, when CRST debuted and I was about halfway through my career. Twenty-one years before then, I learned to perform phacoemulsification from my partners John Graether, MD, and Russell H. Watt, MD, who themselves had learned to perform the procedure at a course directed by Charles D. Kelman, MD, in 1972. Dr. Kelman published his pioneering work1 when I was a junior in high school. I learned more about phacoemulsification from Robert Sinskey, MD; Richard P. Kratz, MD; Thomas T. Mazzocco, MD; and Michael Colvard, MD, FACS, my friend from residency at the Mayo Clinic.
When I joined the Wolfe Eye Clinic, the surgeons there had transitioned from using the original Cavitron/Kelman Phacoemulsifier, which they lovingly called “Big Bertha” (Figure 1), to using the CooperVision 8000 phaco system, which was introduced in 1978. The latter unit had a footpedal that, when pressed, activated ultrasonic energy. We had to assemble the large handpiece, which featured vibrating metal plates and separate hoses from a fluid bath to cool those plates. The circulating nurse turned a knob when we asked for more or less energy. Vacuum was fixed at 47 mm Hg, the same level available with the succeeding 1985 model (CooperVision 9001). This model featured a piezoelectric handpiece and linear phaco power activated with a surgeon-controlled footswitch. It also had high and low vacuum settings for cortex removal.
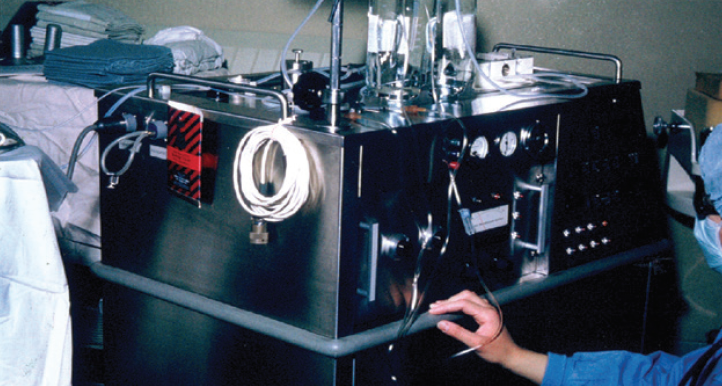
Figure 1. Surgeons at the Wolfe Eye Clinic using the Cavitron phaco system.
With those early machines, we simply debulked the central nucleus by shaving it away and emulsified the peripheral nucleus from the outside in (imagine eating a hamburger that way) while repeatedly prolapsing a new superior edge so that the phaco tip could aspirate new lens dust. With repeated rotations of the nucleus until the entire peripheral edge was eventually shaved away, only the central posterior plate was left to be emulsified and aspirated.
REVOLUTIONARY ADVANCES IN TECHNIQUE AND TECHNOLOGY
With all the revolutionary advances since the birth of phacoemulsification in 1967, who would have thought that we’d be using the same core technology today? Several landmark advances in surgical technique were made in the 1980s and 1990s; these included the development of the continuous curvilinear capsulorhexis,2 divide and conquer,3,4 cortical cleaving hydrodissection,5 phaco chop,6 stop and chop,7 and phaco prechop.8 Phaco chop techniques could be executed safely thanks to the development of Viscoat (Alcon), which protected the cornea,9 and improvements in technology that allowed surgeons to simultaneously control the vacuum level, flow rate, and tip movement.
With Alcon’s introduction of the Legacy 20,000 in 1993, surgeons could control vacuum up to 101 mm Hg and use 0.9-mm–diameter aspiration bypass system tips. Ten years later, the introduction of the Infiniti Vision system (Alcon) allowed surgeons to control vacuum up to 500 mm Hg. The Infiniti also integrated pulse/burst programming, and an upgrade 2 years later permitted torsional tip movement for increased followability10 to help reduce phaco time and balanced salt solution volume. With torsional ultrasound, larger chunks of the nucleus could be separated and aspirated more efficiently.
This system still required small situational adjustments because relatively abrupt changes in dynamics could make the posterior capsule more vulnerable or cause the iris to shudder, decreasing pupil size. The Centurion Vision System (Alcon) replaced the Infiniti in 2013. It’s 0.9-mm Intrepid Aspiration Bypass System Balanced Tip increased emulsification efficiency while minimizing movement of the phaco tip shaft in the incision. Refined computerized blending of torsional and longitudinal tip motions improved the control with which quadrants of all grades could be aspirated. The integrated dual-pump technology of the Intrepid and its programmable IOP and vacuum/flow ramps greatly refined fluidic controls and intraoperative behavior.
TECHNIQUE AND INSTRUMENTATION
My technique has evolved over time, but my goal remains the same: to maximize capsular/zonular integrity and minimize corneal endothelial cell density loss. I have always believed that the machine-calculated total dissipated energy of the phaco tip affects corneal endothelial cell density less than the proximity of the tip and the pieces of nucleus that grind against the cornea.11 (The farther away from the cornea, the better.)
I still create four separate quadrants and hollow out the nucleus as much as possible while it is in situ in the capsular bag. The bulk of each quadrant can be reduced by aspirating as much deep nuclear dust as possible,12 while creating an intracapsular space in which to emulsify the remaining relatively 2D plates of peripheral nucleus. This helps to protect the cornea when larger pieces of nucleus are drawn into the anterior chamber for emulsification. The average rate of endothelial cell density loss for hard cataracts with this technique is 5%.13 The capsule is protected by the physical insulation offered by the nuclear plates underneath the quadrant that is being emulsified.
Until 5 or 6 years ago, I used a customized modified cyclodialysis spatula (Storz SP7-71996, Bausch + Lomb; 0.5 mm reduced to 0.33 mm) or a Connor Wand (Storz E7250; Bausch + Lomb) to tear the posterior nuclear plate after sculpting deep grooves. This maneuver was hard to execute if the nucleus was very soft or hard. Arthur J. Weinstein, MD, reintroduced me to the Akahoshi Prechopper (2267E, Ambler Surgical), which I now use in about 80% of cases. I find this instrument extremely helpful for separating soft quadrants (LOCS III nuclear opalescence and nuclear color [NC] ≤ 3.6).14 For quadrants with greater NC and nuclear opalescence, I use the cyclodialysis spatula or Connor Wand. For very hard or mature cataracts, I even use an Akahoshi prechopper under an OVD after grooving. The instrument is placed curved side down to crack the posterior nuclear plate completely with almost no nucleus displacement (Figure 2; video below). I switch to the epinucleus setting to aspirate enough of the OVD to reestablish irrigation and aspiration before engaging phaco tip energy, thereby preventing thermal damage to the corneal incision.
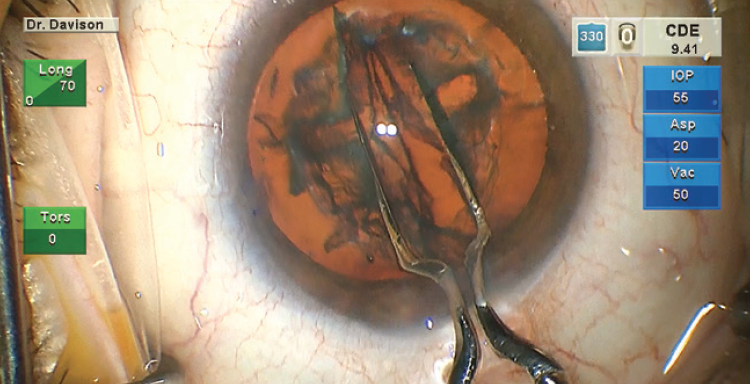
Figure 2. An Akahoshi prechopper is used with the curved side down to crack the posterior nuclear plate.
Optimizing my personal settings on the Centurion provides me with a level of control that gives me confidence in any challenging situation (Figure 3). I am not aggressive in my settings so that I can be proactive rather than reactive. For soft nuclei that resist cracking, the sculpt setting is used to debulk the nucleus, and the epinucleus setting is used to aspirate successive clock hours of peripheral nucleus as it is rotated toward the tip. This rotation often results simply from the aspiration of successive new material at the tip. For firm nuclei, the sculpt setting is used to create grooves. After cracking, the deeper, firm corners are shaved away from each quadrant (Figure 4). Drawing in the first quadrant of soft nuclei (LOCS III NC ≤ 3.6) can be challenging. In my experience, the epinucleus setting provides footpedal-controlled vacuum adjustments with preset vacuum-triggered tip movement15 so that I can gradually draw the first quadrant away from the peripheral capsule. I use the preprogramed vacuum rise time of the quadrant removal setting (Figure 5) and control the amplitude of the phaco tip motion with the footpedal. To improve fine control, the last quadrant or two can be carefully acquired and positioned using the epinucleus setting at any time during quadrant removal. If the nucleus is hard, I will reinject a dispersive OVD, especially before aspirating the last quadrant.
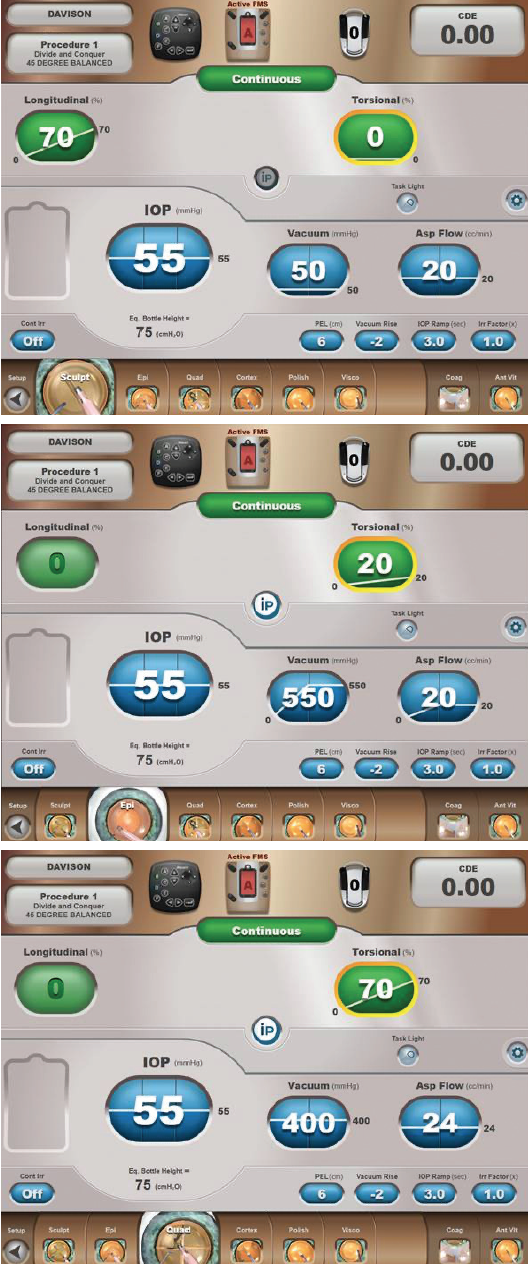
Figure 3. Dr. Davison’s settings for the Centurion.
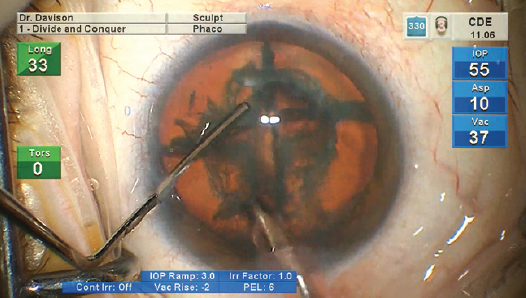
Figure 4. A firm corner is shaved away from the quadrant.
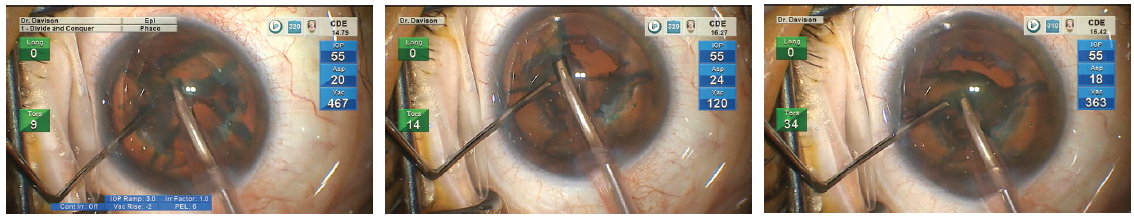
Figure 5. Dr. Davison uses the preprogramed vacuum rise time of the Centurion’s quadrant removal setting.
Figures 1–5 courtesy of James A. Davison, MD, FACS
CONCLUSION
I wish I could trade in my first 10 years of practice for another 10 now, but I know that that’s not going to happen. I’ve been lucky to enjoy so many advances in phacoemulsification. I don’t know how it can get much better, but I know it will.
Congratulations, CRST! You have delivered timely and accurate information and insight that have allowed ophthalmologists such as myself to improve our surgical techniques, practices, and patient outcomes and experiences. I look forward to another successful and meaningful 20 years of this publication.
1. Kelman CD. Phacoemulsification and aspiration–a new technique of cataract removal: a preliminary report. Am J Ophthalmol. 1967;6423-6435.
2. Graether JM. Continuous circular anterior capsulotomy under Healon. Ocular Surgery News. 1986;3(13):2-3.
3. Shepherd JR. In situ fracture. J Cataract Refract Surg. 1990;16(4):436-440.
4. Gimbel HV. Divide and conquer nucleofractis phacoemulsification: development and variations. J Cataract Refract Surg. 1991;17(3):281-291.
5. Fine IH. Cortical cleaving hydrodissection. J Cataract Refract Surg. 1992;18(5):508-512.
6. Nagahara K. Phaco Chop. Film presented at: 1993 ASCRS Annual Meeting; August 26-29, 1993; Los Angeles.
7. Koch PS. Stop and chop phacoemulsification. J Cataract Refract Surg. 1994;20(5):566-570.
8. Akahoshi T. Phaco Prechopper. Film presented at: 1994 ASCRS Annual Meeting; April 9-13, 1994; Boston.
9. Koch DD, Liu JF, Glasser DB, Merin LM, Halt E. A comparison of corneal endothelial changes after use of Healon or Viscoat during phacoemulsification. Am J Opthalmol. 1993;115(2):188-201.
10. Davison J. Cumulative tip travel and implied followability of longitudinal and torsional phacoemulsifaction. J Cataract Refract Surg. 2008;34:986.
11. Graether JM, Davison JA, Harris GW, Watt RH, Widner RR, Sposito VA. Comparison of the effects of phacoemulsification and nucleus expression on endothelial cell density. American Intra-Ocular Implant Society Journal. 1983;420-423.
12. Davison J. Bimodal capsular bag phacoemulsification: a serial cutting and suction ultrasonic nuclear dissection technique. J Cataract Refract Surg. 1989;15:272-282.
13. Davison J. Results of endocapsular phacofracture debulking of hard cataracts. Clinical Ophthalmol. 2015;1233-1238.
14. Chylack LT, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. JAMA Ophthalmol. 1993;111(6):831-836.
15. Davison JA. Two-speed phacoemulsification for soft cataracts using optimized parameters and procedure step toolbar with Centurion Vision System and Balance Tip. Ophthalmology. 2015;1563-1572.
There’s Always Room for Improvement
Himani Goyal, MD

Even after 12 years in practice, I know there is room for greater efficiency in my cataract surgery technique. In my mind, a deliberate flow must be present for surgeons to generate efficiency:
No. 1: Practice;
No. 2: Watch and learn from your peers how to perform new techniques and use new tools; and
No. 3: Review your own videos and note areas for improvement.
Dr. Goyal’s Cataract Surgery Efficiency Milestones
2007 Learning the basics during residency and building confidence as a surgeon
- Wayne L. Scott, MD: “You have control. You can go a little faster now!”
2008 Completing the last 3 months of residency with Michael Nejat, MD, FAAO
- Watch one. Do one. Repeat.
2015 Learning to go faster with confidence in part due to improved chamber stability with the Centurion Vision System (Alcon)
- Watch the video below.
2017 Learning to obliterate dense cataracts with the miLoop (Carl Zeiss Meditec): my last scleral tunnel!
- Watch the video below.
2018 Learning MIGS and subsequently combining MIGS with iStent inject (Glaukos) implantation and Kahook Dual Blade (New World Medical) goniotomy
- Watch the video below.
2021 Working toward reducing transition times from one step to the next
- Learning from friends, including Lorenzo J. Cervantes, MD; Watch the video below.
This process must be repeated continually to ensure that your efficiency does not become stagnant.
If you think about it, this deliberate flow in process is how all surgeons get started. Residents practice what they learn from watching their attendings. As residents become more proficient, they are encouraged to try new techniques and use new tools, and they are critiqued on every step. They go through the deliberate and constant flow of practicing, watching, and reviewing until they become nearly perfect.
Keeping an open mind and being proactive
We are all works in progress concentrating on ways of making cataract surgery more efficient. The key is to share our ideas and learn from each other. New tools and techniques are introduced so often that keeping an open mind and proactively continuing to learn new things is necessary.
Some of the milestones I have achieved that have helped me to become a more efficient cataract surgeon are detailed in the accompanying sidebar. So many of my colleagues have helped to inspire me and develop the surgeon in me, and they continue to motivate me to become better every day. I aim to pass this knowledge on to my residents and motivate others to do the same.
Increasing Efficiency by Minimizing Risks
Björn Johansson, MD, PhD, FEBO

Advances in cataract surgery continuously change the procedure for the better. I am astounded by the number and variety of developments I’ve witnessed since I performed my first complete case in 1990—a successful intracapsular cryoextraction with no evident vitreous loss and a happy, aphakic patient as a result. I transitioned from extracapsular cataract extraction through a large incision to phacoemulsification performed through a 3.5-mm scleral tunnel incision and the implantation of a one-piece, 6-mm PMMA IOL. Not long thereafter, I began performing surgery through a 2.75-mm clear corneal incision and implanting a foldable IOL. Recently, I incorporated advances such as immediate sequential bilateral cataract surgery (ISBCS), intracameral antibiotics, microincisional cataract surgery through a 1.8-mm incision, and preloaded IOLs. To describe how I have increased my surgical efficiency in the past few years is challenging given the breadth of developments in the field.
Most cataract procedures are relatively straightforward. Around 10% of patients, however, present with one or more of the following: limited pupil dilatation, weak zonules, intraoperative floppy iris syndrome, a mature white cataract, a dark brown rock-hard cataract, a history of vitrectomy with or without silicone oil, or a shallow or deep anterior chamber. I work in a tertiary university hospital, and I encounter a large number of highly complicated cataract cases.
A STABLE CHAMBER
Whether the cataract procedure is routine or complex, efficient surgery demands a stable chamber. This can be achieved most easily with a phaco system that allows the surgeon to adjust the ultrasound power and evacuating force system independently on the fly as necessary.
I operate through a 1.8-mm tunnel incision, and I can adjust the evacuating force and ultrasound power independently with the dual-linear footpedal control of the Stellaris Elite Vision Enhancement System (Bausch + Lomb). In Figure 6 and the accompanying video (below), I demonstrate the independent adjustment of the ultrasound energy and vacuum to optimize safety and efficacy during the different surgical phases of a combined cataract and glaucoma surgical procedure.
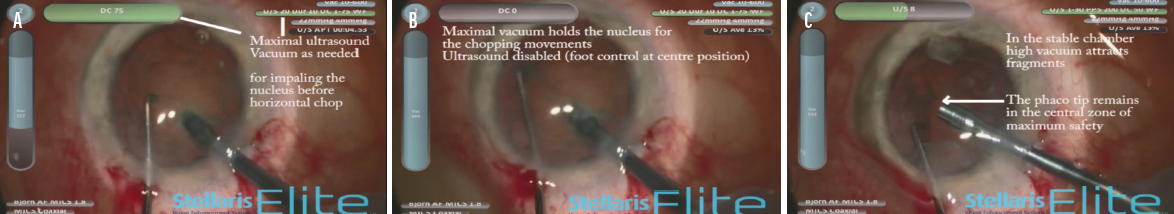
Figure 6. The dual-linear footpedal is used to employ vacuum force (vertical pedal movement, vertical blue gauge) and ultrasound energy (horizontal pedal movement, horizontal green gauge) independently through the surgical phases, including impaling the nucleus before horizontal chopping (A), holding the nucleus with maximal vacuum during the chopping movement (B), and removing the nuclear fragments while the phaco tip is kept steady at the pupil plane in the middle of the anterior chamber (C).
Courtesy of Björn Johansson, MD, PhD, FEBO
The Active Fluidics function of the Stellaris Elite can help to maintain a stable chamber while keeping the IOP low. This is especially advantageous for eyes with advanced glaucoma.
RISK REDUCTION
Minimizing the risk of adverse events during cataract surgery is crucial. For patients with complex cataracts or ocular comorbidities especially, greater safety is associated with greater efficiency. For uncomplicated cases, I favor ISBCS to reduce risks and optimize efficiency. I find that, compared to routine cataract surgery, ISBCS promotes faster visual rehabilitation with the least effort required from both the surgical team and patient. ISBCS is also easier on caregivers because there is only one surgery date rather than two.
Many surgeons have adopted ISBCS during the COVID-19 pandemic. ISBCS has become a more acceptable alternative, and more surgeons recognize the benefits it brings to themselves, patients, and health care systems. The UK Royal College of Ophthalmologists, among others, has issued advice for the safe implementation of ISBCS. With ISBCS, surgery on each eye is a completely separate procedure.
CONCLUSION
Improvements in surgical technique and surgical course, such as ISBCS, have increased efficiency for my cataract patients.
Simplicity, Sophistication, and a Meticulous Surgical Technique
Jason J. Jones, MD
<
When I think back over the past 20 years, I see that phaco technologies that seemed to have incremental improvements at the time had a cumulative and dramatic impact on the safety and efficiency of cataract surgery and on the visual outcomes that the procedure can deliver. My advice to surgeons who want to take their phaco outcomes to the next level is to rely on both simplicity and sophistication.
SIMPLICITY
By the word simplicity, I mean an old-school reliance on accurate biometry, meticulous surgical technique, effective communication, and a careful review of cases.
I strongly recommend making a video recording of every case and routinely reviewing the footage. When reviewing my own cases, I look for unnecessary movements, mistakes, and vulnerabilities, and I assess how much energy and fluid was used. I also recommend watching videos shared by master surgeons.
Investing time in the refinement of surgical technique and the improvement of surgical skills can improve outcomes and efficiency. Learn to use venturi fluidics, transition to more aggressive vacuum settings with a venturi system (Figure 7), and—if the phaco system permits—learn to switch between the venturi and peristaltic modes to take advantage of the benefits of each during a single procedure. In my experience, industry representatives can be helpful when surgeons want to customize and refresh their phaco settings as they alter their techniques.
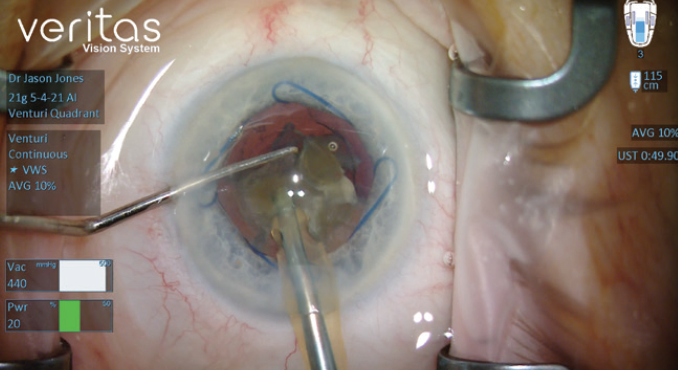
Figure 7. Dr. Jones’ venturi vacuum settings.
Courtesy of Jason J. Jones, MD
SOPHISTICATION
Invest in new phaco technology. It is easy to become comfortable with a workhorse device and put off updates, but a new device can provide significantly better fluidics control, chamber stability, ultrasound energy efficiency, and ergonomic comfort. I recently began using the Veritas Vision System (Johnson & Johnson Vision), which I have found to offer several advantages over my previous machine. One of these is advanced infusion, which increases irrigation flow with pressurized air (22 mm Hg) to provide the equivalent of an extra 30 cm of infusion bottle height. As I transition to footpedal positions 2 and 3, the system automatically increases the irrigation pressure to deepen the anterior chamber and expand working space. The advanced tubing system minimizes the variability of fluid volume in the tubing under maximum vacuum, which can reduce line surge. I have been best able to take advantage of these chamber stability features by using a smaller-bore, 21-gauge phaco tip rather than a 20-gauge tip.
Pay attention to technology that can reduce physical and mental stress on the surgeon, staff, and patients. The Veritas emits lower-pitched tones, offers the option of dark background screens, and has a small and light swivel handpiece. The distal end of the handpiece can rotate up to 220º without movement of the surgeon’s wrist, which can make the handpiece easier to hold and manipulate. The proximal end remains stationary, so the tubing and power cables drape smoothly away; this separation helps me to interact with cataract incisions more gently.
My practice has also purchased automated reclining surgical chairs made by UFSK-OSYS that can be moved easily between the preoperative holding area and the OR. This can reduce the staff’s workload and increase patient safety and comfort.
Adoption of Combined Microincisional Cataract and Vitrectomy Surgeries
Mitrofanis Pavlidis, MD

For the past 5 years or more, combined microincisional phacovitrectomy (MICVIT) has been my standard technique for the surgical management of vitreoretinal disorders and concomitant cataract. This technique offers multiple performance and outcome benefits (see MICVIT: Multiple Benefits Maintained).
MICVIT: MULTIPLE BENEFITS MAINTAINED
- Exceptional chamber stability throughout both phaco and vitrectomy maneuvers
- Optimal visualization during vitrectomy with avoidance of cornea opacity
- Prevention of accidental tissue damage with small-gauge instrumentation and flow control
- Minimal trauma and reduced rates of postsurgical hypotony and anterior chamber irritation/inflammation
- Optimal patient comfort with no suturing
- Low to no surgically induced astigmatism
- Faster postsurgical visual rehabilitation compared to two separate procedures
Technical innovations involving sophisticated I/A technologies, software-based simulation, and next-generation materials spurred rapid developments in cataract and vitrectomy surgery over the past decade. Further, advances in instrumentation and vitreoretinal surgery systems have inspired surgeons, myself included, to evaluate and standardize newer techniques and vitreoretinal maneuvers for improved surgical performance. These evolutions have directly influenced my surgical practice and the evolution of MICVIT into one of my favorite techniques. MICVIT involves performing cataract surgery through a 1.8-mm incision and a 27-gauge vitrectomy.1
SURGICAL STEPS
A 27-gauge valved trocar is placed in the inferior temporal quadrant 4 mm from the limbus. A 1.8-mm clear corneal incision is made at the 10 clock position. The same phaco knife is then used to make a 1.2-mm paracentesis incision. The angulation of the tip (approximately 90º) combined with the small incision size minimizes surgically induced astigmatism.
Once the incisions have been created, an OVD is injected into the anterior chamber. Creating only two small corneal incisions helps to prevent the OVD from escaping during subsequent surgical maneuvers. A capsulorhexis is then performed with microtip capsulorhexis forceps such as the Hattenbach (Geuder) and Mohr (DORC) that are suitable for phaco procedures using 1.8-mm incisions. The precise distal end of these forceps allows the surgeon to comfortably perform additional capsulorhexis maneuvers that may be required, including the redirection of an errant tear or the creation of a posterior capsulorhexis.
PREFERENCES
I use a beveled 30º 1.8-mm coaxial phaco tip (Eva EquiPhaco, DORC) because I find that it improves my ability to direct ultrasound energy and thus avoid damaging the endothelium. To improve the efficiency of phacoemulsification, the phaco tip is rotated so that peripheral nuclear fragments can be aspirated. Additionally, I find that a wider phaco tip design provides improved holdability for high-efficiency emulsification, even with sub–2-mm phaco surgery.
Vacuum formation with the phacovitrectomy machine is based on the dual-mode valve timing intelligence aspiration system of the Eva. This provides a linear maximum vacuum level of 680 mm Hg in 0.3 seconds, a considerably faster vacuum rise time than with traditional venturi systems. In flow control mode, the surgeon can determine a precise aspiration flow rate, eliminating pulsation. Further, the valve timing intelligence pump continuously adjusts vacuum for reliable aspiration, ensuring excellent fluidic and retinal stability throughout the procedure. A pressurized balanced salt solution bottle provides intraocular irrigation control.
I prefer a one-piece IOL with a four-point haptic design, which is available across a range of lens models.
If propulsion and shallowing of the anterior chamber occurs, a 5- to 10-second vitrectomy performed through the trocar can release posterior pressure and deepen the anterior chamber. At the end of phacoemulsification, almost no hydration of the incision is performed. The cornea should be clear, allowing a peripheral vitrectomy to be performed, and the chamber should be stable with no need for suturing. A 27-gauge high-flow infusion line is connected to the preset trocar, and two additional trocars are placed 3.5 mm from the limbus at the 4 and 10 clock positions for the left eye or the 8 and 2 clock positions for the right eye. A smooth flow-controlled 27-gauge vitrectomy is then performed using a double-action 2D cutting vitrectome (TDC, DORC).2
In eyes without capsular support, the same trocar setup can be used for sutureless intrascleral IOL fixation.3-5 A demonstration of this technique with a trocar cannula system featuring a push-fit infusion connection (Eva Aveta, DORC) may be viewed by watching the video below.
1. Pavlidis M, Körber N, Höhn F. Surgical and functional results of 27-gauge vitrectomy combined with coaxial 1.8 mm microincision cataract surgery: a consecutive case series. Retina. 2016;36(11):2093-2100.
2. Pavlidis M. Two-dimensional cutting (TDC) vitrectome: in vitro flow assessment and prospective clinical study evaluating core vitrectomy efficiency versus standard vitrectome. J Ophthalmol. 2016;2016:3849316.
3. Gabor SG, Pavlidis MM. Sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2007;33(11):1851-1854.
4. Scharioth GB, Prasad S, Georgalas I, Tataru C, Pavlidis M. Intermediate results of sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2010;36(2):254-259.
5. Pavlidis M, de Ortueta D, Scharioth GB. Bioptics in sutureless intrascleral multifocal posterior chamber intraocular lens fixation. J Refract Surg. 2011;27(5):386-388.
A High-Quality Phaco Machine
Vittorio Picardo, MD

As in most parts of the world, cataract surgery is an outpatient procedure in Italy. Most ophthalmologists who work in a hospital setting often are not able to meet with the patients on the surgical lists in advance of surgery, and the order of individual surgeries is not organized based on the perceived difficulty of the procedures. Cases of soft or hard cataracts and small pupils, for example, are peppered throughout each surgical session. Surgeons therefore must be prepared to complete surgical planning for each procedure on the fly. To prevent surgical complications from occurring, I rely on high-quality surgical instruments and devices, including knives, forceps, and OVDs. At the top of my list, however, is the phaco machine.
The R-Evolution phaco system (Optikon) helps me to perform safe and high-quality surgery routinely. With this platform, I can customize my surgical settings and parameters, which helps me to optimize the procedure and promotes exceptional surgical quality. I can also program separate settings for a variety of surgical situations, which is helpful when I am operating on cataracts of different grades and patients with differing ocular anatomy. Additionally, I can switch between peristaltic and venturi pumps.
PERSONAL SETTINGS
When the density of the cataract is soft to medium, I do not sculpt the nucleus, and I do not use the dual-linear footpedal function. My routine parameters with a peristaltic pump are as follows:
- Bottle height, 80 cm;
- Ultrasound power, 35%;
- I/A flow rate, 30 mL/min; and
- Vacuum, 300 mm Hg.
I use the same I/A flow rate with or without ultrasound. These settings help me to use a divide-and-conquer or stop-and-chop technique in a controlled fashion in about 80% of cases.
In certain complex situations such as an eye with a small pupil, I modify my settings and use a venturi pump. This combination helps me to keep the phaco tip in the center of the pupil. I can then move the nucleus pieces toward the tip. My routine parameters with a venturi pump are as follows:
- Bottle height, 80 cm;
- Ultrasound power, 20%; and
- Vacuum, 350 mm Hg.
ADDITIONAL PEARLS
I have achieved good functional results with flared phaco tips. Optikon has a research laboratory in Rome, and I have collaborated with researchers both to develop settings for the company’s existing phaco tips and to produce new tip models. This work has improved my phaco efficiency.
The R-Evolution system can also be used for vitreoretinal surgery. If the posterior capsule ruptures during cataract surgery, I can immediately switch settings and complete a posterior vitrectomy safely and efficiently.
The Importance of Improved Phaco Tip Designs
Armin Wolf, MD, FEBO

Both surgeons and patients have benefitted from the myriad advances in cataract surgery that have been achieved in the past few years. Access to advanced diagnostics, a broader range of IOLs such as trifocal and extended depth of focus lenses, and modern surgical platforms have increased the precision of cataract surgery and enhanced postoperative results. Greater attention to patients’ specific needs and a more individualized approach to surgery have improved the patient experience.
The cataract procedure itself has undergone many changes. Many surgeons use a femtosecond laser to increase the efficiency of the procedure. In my practice, however, I rely on a modern phaco machine to perform safer, more streamlined cataract surgery.
Improved phaco tip designs have contributed to the increased efficiency of the CataRhex 3 phaco machine (Oertli). The unit’s easyPhaco technology promotes safe and efficient emulsification and aspiration of the nucleus and increased anterior chamber stability. The easyPhaco tip features a beveled design, which I find makes drawing nuclear fragments to the phaco tip simpler. Further, optimized fluidics, which is based on physics, delivers more linear flow in the anterior chamber during phacoemulsification.
Thanks to these improvements in tip design, I was able to decrease my incision size to between 1.6 and 1.8 mm. My effective phaco time is approximately the same as it is with a larger incision size.
Phaco machines with controllable pump systems can also increase efficiency. Many modern units allow surgeons to switch between peristaltic and venturi pumps, which control fluidics mechanically or by an air current, respectively. The CataRhex 3 also includes a unique pump system, the SPEEP pump, and I can quickly switch between the peristaltic and the SPEEP pumps depending on the procedure.
SPEEP mode combines the positive effects of a flow-controlled peristaltic pump with the direct response of a venturi pump system. With the SPEEP pump, flow and vacuum may be controlled independently of each other, which I find gives me greater control over fluidics. The preset flow rate enhances anterior chamber stability, which is essential for safe phacoemulsification. This is especially important when increased and more advanced settings are used. I can control vacuum settings to achieve a fast response time and enhance the overall performance of the pump while maintaining subtle control of the anterior chamber. Even in the setting of complete occlusion, I can control the vacuum independently.
CONCLUSION
The development of more efficient phaco tips and controllable pump systems has helped me to increase the efficiency of my cataract surgery technique. It also helps me to perform surgery safely, even in complex cases.




