Case Presentation
A 48-year-old woman presented for routine refractive surgery. Her BCVA was 20/20 OU with a manifest refraction of -3.50 +0.25 x 076º OD and -2.75 D sphere OS. Pachymetry readings were 553 µm OD and 558 µm OS. Pupil size was 4 mm OU by Colvard pupillometry. Keratometry readings were 46.50/45.37 D @ 164º OD and 46.75/45.62 D @ 6º OS with a normal pattern. The patient did not elect monovision and was scheduled for routine bilateral LASIK surgery.
Thirty minutes before surgery, oral diazepam (Valium, Roche) 5 mg was administered. To anesthetize the patient’s eyes, one drop of proparacaine was administered before the sterile preparation, and a second drop was administered before the keratectomy. LASIK was performed using a Visx S3 ActiveTrak laser and an Amadeus microkeratome (both devices no longer available).
An open-wire Lieberman speculum was used to hold open the eyelids. Because of blepharospasm and tight orbits, difficulty placing suction was encountered.
How would you proceed? What are other options for managing tight orbits? At what point would you consider aborting LASIK and performing an alternative procedure such as PRK? If possible, would you change or choose another microkeratome? Which microkeratome would you prefer to use in a small orbit?
—Case prepared by Y. Ralph Chu, MD
WHAT I DID IN and my thoughts from 2001: Y. RALPH CHU, MD
One of the first steps I take when confronted with a tight orbit is to ensure that the patient is relaxed. I focus on controlling the tone of my voice, which is sometimes called vocal local, and calmly describe what is happening. This not only calms the patient, but it also alerts the staff members to the tense situation at hand so they can focus on controlling a potentially difficult situation. In this case, the patient received diazepam preoperatively, so, despite her blepharospasm, she was relaxed during the entire process. Next, I focus on the lid speculum. I ensure that it is maximally open and gently and repeatedly apply the suction ring. If this is not feasible, I consider removing the lid speculum. If a solid-blade speculum is used initially, switching to an open wire speculum can be a helpful intermediate step before attempting to perform the procedure without a speculum.
After removing the suction ring, I reassess the patient’s orbital anatomy and carefully distinguish between a narrow palpebral fissure and a tight bony orbit. I also examine the depth of the orbit, which can affect suction ring placement. If there is adequate bony orbital exposure, performing the keratectomy without a lid speculum may be effective. Depending on the microkeratome, protecting the lids with Nexcare Steri-Strips (3M) or drapes may prevent lid margin damage during the keratectomy. The Amadeus unit that I used at that time had a protected space design to permit routine usage without a lid speculum, making it an attractive choice for managing tight orbits.
If the globe is deep-set within a tight bony orbit, the surgeon may need to consider more drastic measures, such as a lateral canthotomy or a retrobulbar injection of anesthesia to help proptosis and elevate the globe out of the orbit. In these extreme situations, I usually consider aborting the LASIK procedure and discussing an alternative procedure with the patient, depending on his or her refractive error. In similar patients with low myopia, PRK would be an excellent option. For higher degrees of refractive error, a phakic IOL or a lens extraction procedure may be necessary. In either scenario, the best choice is to abort the current procedure and discuss options with the patient.
In this case, the lid speculum was removed. With adequate suction ring placement, the procedure was performed carefully without a lid speculum in place. A sterile, dry 4 x 4–inch sponge was used to help retract the lids and better expose the globe. I also ensured that the lid margin was completely clear of the suction ring to prevent pseudosuction. That is, the suction ring in a tight orbit can sometimes grab the lid margin, simulating adequate suction, but this pseudosuction results in a poor flap. For managing a small orbit, I would choose a microkeratome that could be used without a lid speculum. I would also choose one with a gearless system because gears can jam on lashes and lids.
Surgery on both eyes was performed on the same day using the same technique. On the first postoperative day, the patient’s UCVA was 20/25 OU. The refraction in each eye was -0.25 D sphere. A slit-lamp examination revealed temporal subconjunctival hemorrhaging in the right eye, but the cornea was otherwise clear. The flap in each eye was well positioned. The patient had an uneventful postoperative course, and at her last postoperative visit, her BCVA was 20/20 OU with a refraction of -0.50 +0.50 x 086º OD and -0.25 +0.50 x 090º OS. The subconjunctival hemorrhage resolved itself, and the corneas were clear.
WHAT I WOULD DO IN 2021
Y. Ralph Chu, MD

Upon reflecting on the article that I wrote for the debut issue of CRST, I cannot help but think of the old saying, “The more things change, the more they stay the same.” During the past 20 years, refractive surgeons transitioned from using mechanical microkeratomes to femtosecond lasers for the creation of LASIK flaps. A completely laser-driven flapless procedure, SMILE, was also introduced. The basic principles of treating patients who have blepharospasm, a narrow palpebral fissure, and difficulty tolerating the placement of suction, however, remain the same.
My current protocol begins during the preoperative evaluation and includes an analysis of the orbital anatomy that focuses on the bony orbits and an assessment of the patient’s ability to tolerate the suction ring of the laser. My findings determine whether the patient is a potential candidate for SMILE, LASIK, or surface ablation or if refractive surgery is contraindicated.
In my opinion, modern femtosecond lasers have improved the safety of the LASIK procedure. Lower suction pressure and curved patient interfaces have also helped make the procedure more comfortable for patients. Many of the intraoperative techniques that were described in my original article such as canthotomies, retrobulbar blocks, and peribulbar blocks are now largely unnecessary. If I am not able to obtain adequate suction using a femtosecond laser to create the flap, I now have a lower threshold for converting to surface ablation. Patients are counseled about this prospect before a procedure is considered.
It is amazing to see how far technology for LASIK surgery has advanced. Femtosecond lasers can create flaps of excellent quality and improve safety for both patients and surgeons.
Luke Rebenitsch, MD

A tight orbit is a common issue for refractive surgeons. In this situation, anatomy and anxiety must be addressed simultaneously. The first thing to remember is that a relaxed patient has a more open orbit. I therefore provide diazepam to such patients. It must be remembered that diazepam has an average peak plasma time of 1 to 1.5 hours, so an efficient surgeon may benefit from waiting longer before starting surgery on an anxious patient. I also provide verbal anesthesia in the form of continuous conversation, and the background music is chosen by the patient.
Thankfully, technology has improved since the publication of Dr. Chu’s original article. Refractive surgeons no longer use mechanical microkeratomes but instead use femtosecond lasers. I prefer the Femto LDV Z6 (Ziemer Ophthalmic Systems) for its low energy, high speed, and versatility. This laser docks on the sclera, and the patient must be adjusted to a chin-up and opposite-leaning position. The laser head can also be rotated to fit better in the orbit. An alternative is the VisuMax (Carl Zeiss Meditec), which docks itself onto the cornea and has a smaller patient interface. Thousands of cases have been performed with the VisuMax without a report of an orbit too tight for comfortable docking. Surgeons using this system, however, should keep in mind that verbal coaching may be of greater importance given its lower suction compared to the Z6 (Figures 1 and 2).
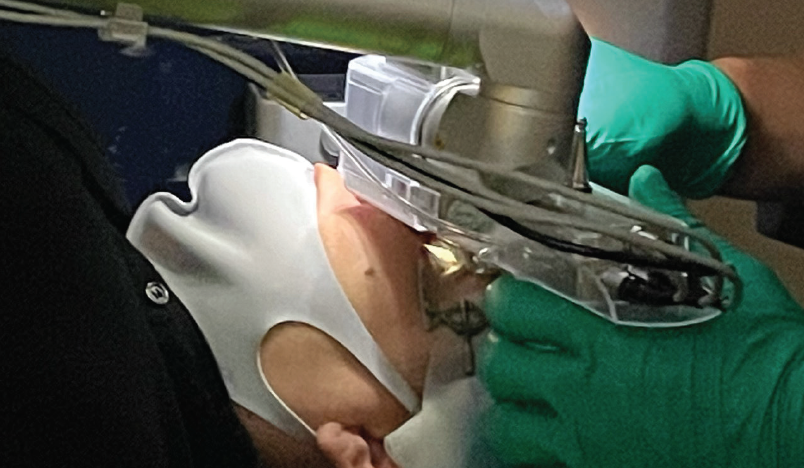
Figure 1. Femto LDV Z6 docking. Note the larger patient interface relative to the VisuMax (Figure 2) and the rotated laser head.
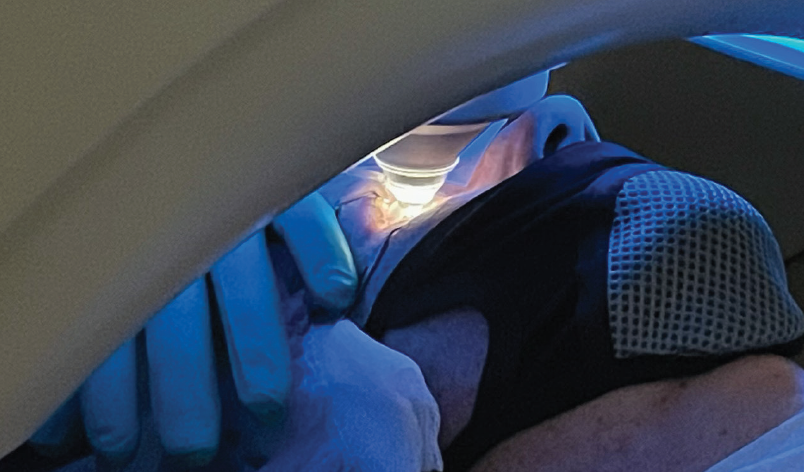
Figure 2. VisuMax docking. Note the smaller patient interface compared to the Femto LDV Z6 (Figure 1).
Courtesy of Luke Rebenitsch, MD
Fortunately, modern technology has made alternatives such as lateral canthotomies and conversion to PRK rarely necessary.
Karl G. Stonecipher, MD, and Alan R. Faulkner, MD

We have discussed this issue often over the years. One of us (A.R.F.) has a large Asian patient population with narrow lid fissures, whereas the other (K.G.S.) has many patients with deep-set eyes. These are two distinct issues: The first involves the eyelids, and the second involves the orbital rim.
Super-careful positioning of the patient is important so that the chin is up and to the left for surgery on the right eye and vice versa. Mechanical microkeratomes are bulkier and can be a challenge to use in patients who have deep sockets and small orbital rims. We abandoned mechanical microkeratomes in 2002 and never looked back. Having access to both a WaveLight FS200 femtosecond laser (Alcon) and an iFS laser (Johnson & Johnson Vision) is a luxury, but, surprisingly, the use of each is advantageous for different anatomic issues.
We cannot overemphasize how relaxed the patient should be. Anxiety contributes to eyelid squeezing, so sedation using an antianxiety pharmaceutical such as alprazolam (Xanax, Pfizer) or diazepam can be helpful.
When there is an issue with the orbital rim, a lid speculum can go only so far before it contacts bone. By comparison, a narrow lid fissure usually permits slightly more stretch with a speculum. Another trick to relax a patient with extremely challenging anatomy is a lid block. Blocking the lid with short-acting lidocaine 1% without epinephrine can achieve relaxation and additional room for surgical maneuvers. For one of us (K.G.S.), lateral canthotomies were required during LASIK after a lid block in two eyes (two patients) out of more than 80,000 cases. In each of these cases, because the eyelids were already numb, the lateral canthus was clamped, and a small lateral canthotomy was made to place the mechanical microkeratome. I (K.G.S.) have never had to use this maneuver since transitioning to a femtosecond laser.
Obtaining informed consent for both PRK and LASIK before the administration of sedation is wise, and it has been our practice for years for patients similar to this one. In this way, if PRK becomes necessary because a LASIK flap cannot be created, it is not necessary for the patient to return at a later date for PRK.
Keith A. Walter, MD, FACS

Dr. Chu handled this case exactly the way I would have handled it 20 years ago. Even today, I would make only a few minor alterations. First, my days of using a microkeratome are long past. I now create LASIK flaps with a WaveLight FS200 femtosecond laser. Thankfully, this laser performs well in patients with small, deep-set bony orbits. I always use an open wire speculum, which I find accommodates 99% of all orbits. From time to time, however, like Dr. Chu, I forego a speculum for those few patients with exceptionally small orbital entries. I also find that positioning the head with equal scleral show inferiorly and superiorly, so that the suction ring fits properly around the limbus, is tremendously helpful when dealing with a tight fit. Using this technique and laser interface, I have had only one eye that required me to abort a LASIK procedure and convert to PRK in the past 15 years.
I agree that relaxing these often highly anxious patients is a key objective. Initially I used diazepam for this purpose, but I found that the drug’s effect was not sufficient for every patient and that the agent occasionally caused nausea. I switched to lorazepam (Ativan, Pfizer), which I found to be more effective but still insufficient at times. Currently, my patients receive alprazolam 1 mg 1 hour before surgery to help with reducing anxiety and inducing sedation. Administration of this drug also ensures that the patient will go home and nap instead of being active after surgery.




