Much has changed over the past 20 years in eye care, cataract surgery, and especially endophthalmitis prevention. Every year, cataract surgery is performed more than 10 million times worldwide and more than 4 million times in the United States alone.1 It is among the safest procedures performed today. Even the best surgical outcomes, however, can be undone by postoperative endophthalmitis (POE)—arguably the most feared and devastating ocular surgical complication.
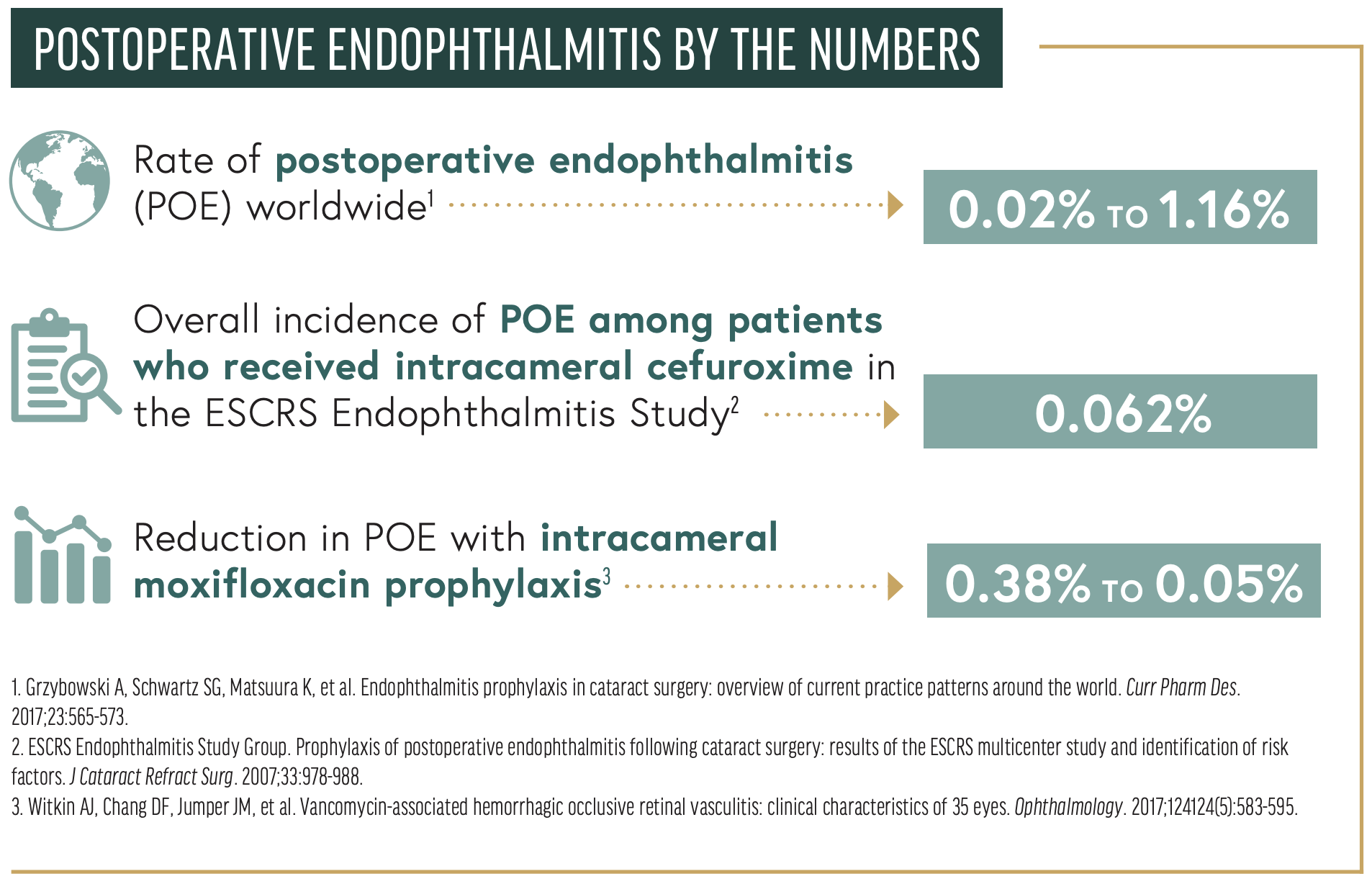
The incidence of POE has not changed much since 2001. The rate worldwide ranges from 0.02% to 1.16%.2 According to the AAO Intelligent Research in Sight Registry (IRIS), the incidence of POE in the United States from 2013 to 2017 was 0.04%. Identifying risk factors and methods of reducing the rate of POE is critical to implementing prophylactic measures and increasing the success of cataract surgery.
PREVENTION
Preoperative considerations. All tried-and-true considerations historically relied on should still be top of mind today. Preexisting ocular surface conditions that are associated with a higher microbial burden or that impair reepithelialization of surgical incisions, including blepharitis, dry eye, exposure conjunctivitis, hordeola, and canaliculitis, should be managed preoperatively.
Surgical preparation. Povidone-iodine 5% solution should be applied to the conjunctiva and cul-de-sac for 2 to 5 minutes and povidone-iodine 5% to 10% detergent to the periocular skin to achieve microbial clearance of the entire periocular surgical area. The lids, lashes, and nasolacrimal system must be isolated by meticulous draping. In prospective clinical trials, topical antibiotic use pre- or postoperatively was not statistically significantly effective at reducing the risk of POE; there is, however, some surrogate evidence that this regimen can kill periocular bacteria.1,3-5
Intraoperative measures. There remains no consensus on the preferred drug, dosing, timing, and modality of application of an antibiotic prophylaxis for POE. The three most common modalities include topical eye drops, subconjunctival injection, and the addition of antibiotics to the irrigating fluid. None of these, however, has demonstrated efficacy in a randomized, placebo-controlled, prospective clinical trial.
INTRACAMERAL ANTIBIOTICS: BENEFITS, RISKS, and alternatives
Benefits. The biggest advance in POE prophylaxis since 2001 is the use of intracameral antibiotics. Unfortunately, this measure is largely inaccessible to surgeons practicing in the United States because a commercial solution is not approved by the FDA.
In 2006, the ESCRS Endophthalmitis Study Group published the results of a prospective, randomized, partially masked, multicenter study comparing the intracameral use of an antibiotic (cefuroxime) to the perioperative use of a topical antibiotic (levofloxacin). The control group, which received topical antibiotics or no antibiotics preoperatively, had a POE rate consistent with the higher end of the worldwide incidence of 0.345%. In comparison, the overall incidence of POE among patients who received intracameral cefuroxime was 0.062%, a 4.92-fold reduction in the incidence of POE. The difference was statistically and clinically significant.6 The addition of perioperative topical drops suggested a possible adjunctive benefit, but the difference was not statistically significant.
Numerous retrospective studies published since the ESCRS’ landmark study have shown a reduction in POE rates with the adoption of intracameral cefuroxime.7-13
Risks. The risks of intracameral cefuroxime also warrant some attention. Dilutional errors have been associated with toxic anterior segment syndrome (TASS) and, if the TASS is severe, with vision loss from corneal decompensation, glaucoma, and/or severe macular edema.14,15 One catastrophic systemic complication reported in the literature is anaphylaxis to the cephalosporin drug.16-19 A careful allergy history is therefore advisable before the use of this prophylactic agent is considered.
Some reports have raised concern about cefuroxime’s limited spectrum of coverage, potentially making it a less favorable antibiotic choice. Intracameral doses can exceed the minimum inhibitory concentrations of many common intraocular inocula. Findings from the LV Prasad Institute and Swedish National Cataract Surgery Database, however, demonstrated that cefuroxime had less efficacy against Gram-negative isolates and methicillin-resistant Staphylococcus aureus (MRSA).20,21 This finding is particularly important because vision loss from gram-negative endophthalmitis is devastating, and the number of reports of MRSA POE is increasing.
Intracameral vancomycin has also been used to prevent POE. This approach has largely been abandoned because of its association with hemorrhagic occlusive retinal vasculitis, a type 3 hypersensitivity reaction that is more destructive than POE. In one study, about two-thirds of affected patients had a final visual acuity of 20/200 or worse, and about one-fifth had no light perception after cataract surgery and the intracameral instillation of vancomycin.22 Furthermore, more than half of patients who experience hemorrhagic occlusive retinal vasculitis will rapidly develop neovascular glaucoma.22 The AAO, FDA, and ASCRS have all advocated against the use of vancomycin as an intraocular prophylactic agent.
Other alternatives. Compared to cefuroxime and vancomycin, moxifloxacin, a fourth-generation fluoroquinolone, has multiple proposed advantages, including a broader spectrum of activity that includes Pseudomonas and community-acquired MRSA. Moxifloxacin is a concentration-dependent, rapidly bactericidal agent that is formulated as an injectable intracameral agent. It is less toxic to intraocular structures than cefuroxime and vancomycin. An in vitro study by Libre et al demonstrated that the most common gram-positive and gram-negative strains that cause POE were eradicated by high-dose moxifloxacin, whereas vancomycin and cefuroxime were less effective.23
A prospective, placebo-controlled, randomized controlled trial found a sevenfold reduction in POE (0.38%–0.05%) with intracameral moxifloxacin prophylaxis compared to no intracameral moxifloxacin.22 Follow-up retrospective studies have demonstrated a three- to sixfold reduction in POE rates when topical moxifloxacin was replaced by intracameral moxifloxacin.24-27 The concentration of safely instilled intracameral moxifloxacin reported in the peer-reviewed literature ranges from 50 to 500 µg/µL without any incident of TASS.28,29
Aravind Eye Hospitals have used 500 µg/0.1 mL moxifloxacin in more than 2 million cases.24-27 This delivery method is easy to use because it is taken directly from a commercially available preparation. Reflux or leakage from corneal incisions upon application, however, can reduce the final amount of drug and, theoretically, its bactericidal effect. The dilution of moxifloxacin (150 µg/0.1 mL) has been proposed.30,31 With this strategy, 0.4 to 0.6 mL of moxifloxacin 150 mg/0.1 mL is used to replace the fluid within the anterior chamber entirely and hydrate the paracentesis. These drug concentrations have been studied in vitro and optimized after an evaluation of cases in which POE occurred despite intracameral moxifloxacin use. There was no proven reduction in POE with either of these two methods of intracameral moxifloxacin delivery.
Biocompatibility, intraocular safety, and lack of toxicity have been demonstrated with a wide range of moxifloxacin concentrations. A prospective, placebo-controlled study by Melega et al found no difference in visual acuity, endothelial cell count, central corneal thickness, and IOP when intracameral moxifloxacin was used.32 Matsuura et al extended observations to 3 years after exposure to intracameral moxifloxacin, and they reported no adverse events and no difference in foveal thickness.33
Moxifloxacin hydrochloride ophthalmic solution with additives such as xanthan gum, sorbitol, or tyloxapol has been reported to incite TASS,34 and the branded topical formulation (Moxeza, Alcon) was discontinued. There have been reports, however, of TASS in eyes that received generic moxifloxacin containing these excipients.35 Caution should be exercised if using a generic antibiotic.
Intracameral antibiotics have been used in conjunction with povidone-iodine in landmark studies on POE prophylaxis.6 When intracameral antibiotics are used off-label in the United States, it is often in conjunction with perioperative topical antibiotics. In a 2014 ASCRS survey, 97% of respondents prescribed postoperative antibiotic eye drops.36 Topical antibiotics are usually dosed four times a day for 5 to 7 days postoperatively.
CONCLUSION
POE is by all measures one of the most devastating complications of any ocular procedure. Many attempts have been made to reduce the risk of endophthalmitis after anterior segment surgery, and most of these have involved antibiotics. In the past 2 decades, the optimal choice of drug, timing, placement, and concentration has become clearer, but more information is required.
Eye surgeons are on the cusp of further reducing POE rates by optimizing the use of antibiotics and other strategies. Current recommendations to reduce the incidence of POE include applying the antiseptic povidone-iodine 5% to the conjunctiva; meticulous draping of the lids, lashes, and lacrimal system; and creating watertight incisions.
The use of intracameral antibiotics is the greatest advance in POE prophylaxis in the past 20 years. The implementation of this strategy can reduce the rate of infection fivefold. Imagine the advances that will occur in the next 20 years. It is to be hoped that POE can be eliminated in the next 20 years and we’ll be talking about postoperative endophthalmitis as something that occurred in our specialty’s past.
1. Grzybowski A, Schwartz SG, Matsuura K, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns around the world. Curr Pharm Des. 2017;23:565-573.
2. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988.
3. Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 35 eyes. Ophthalmology. 2017;124124(5):583-595.
1. Pershing S, Lum F, Hsu S, et al. Endophthalmitis after cataract surgery in the United States: a report from the Intelligent Research in Sight Registry, 2013-2017. Ophthalmology. 2020;127(2):151-158.
2. Grzybowski A, Schwartz SG, Matsuura K, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns around the world. Curr Pharm Des. 2017;23:565-573.
3. Speaker MG, Milch FA, Shaf MK, et al. Role of external bacterial flora and the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639-649.
4. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109:13-24.
5. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
6. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988.
7. Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28(6):977-981.
8. Yu-Wai-Man P, Morgan SJ, Hildreth AJ, et al. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:447-451.
9. Barreau G, Mounier M, Marin B, et al. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg. 2012;38:1370-1375.
10. Myneni J, Desai SP, Jayamanne DG. Reduction in postoperative endophthalmitis with intracameral cefuroxime. J Hosp Infect. 2013;84:326-328.
11. Beselga D, Campos A, Castro M, et al. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5-year study. Eur J Ophthalmol. 2014;24:516-519.
12. Rahman N, Murphy CC. Impact of intracameral cefuroxime on the incidence of postoperative endophthalmitis following cataract surgery in Ireland. Ir J Med Sci. 2015;184.
13. Röck T, Bramkamp M, Bartz-Schmidt KU, et al. Using intracameral cefuroxime reduces postoperative endophthalmitis rate: 5 years experience at the University Eye Hospital Tübingen. Klin Monbl Augenheilkd. 2014;231:1023-1028.
14. Sharma S, Sahu SK, Dhillon V, et al. Reevaluating intracameral cefuroxime as a prophylaxis against endophthalmitis after cataract surgery in India. J Cataract Refract Surg. 2015;41:393-399.
15. Van der Merwe J, Mustak H, Cook C. Endophthalmitis prophylaxis with intracameral cefuroxime in South Africa. J Cataract Refract Surg. 2012;38:2054.
16. Wong DC, Waxman MD, Herrinton LJ, et al. Transient macular edema after intracameral injection of moderately elevated dose of cefuroxime during phacoemulsification surgery. JAMA Ophthalmol. 2015;133:1194-1197.
17. Parssinen O. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50 mg/mL intracameral cefuroxime. Acta Ophthalmol. 2012;90:e153-e154.
18. Moisseiev E, Levinger E. Anaphylactic reaction following intracameral cefuroxime injection during cataract surgery. J Cataract Refract Surg. 2013;39(9):1432-1434.
19. Villada JR, Vicente U, Javaloy J, Alió JL. Severe anaphylactic reaction after intracameral antibiotic administration during cataract surgery. J Cataract Refract Surg. 2005;31(3):620-621.
20. Sharma S, Sahu SK, Dhillon V, Das S, Rath S. Reevaluating intracameral cefuroxime as a prophylaxis against endophthalmitis after cataract surgery in India. J Cataract Refract Surg. 2015;41(2):393-399.
21. Behndig A, Cochener B, Güell JL, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns in 9 European countries. J Cataract Refract Surg. 2013;39(9):1421-1431.
22. Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 35 eyes. Ophthalmology. 2017;124124(5):583-595.
23. Libre PE, Mathews S. Endophthalmitis prophylaxis by intracameral antibiotics: in vitro model comparing vancomycin, cefuroxime, and moxifloxacin. J Cataract Refract Surg. 2017;43(6):833-838.
24. Haripriya A, Chang DF, Namburar S, et al. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123:302-308.
25. Haripriya A. Antibiotic prophylaxis in cataract surgery – an evidence-based approach. Indian J Ophthalmol. 2017;65:1390-1395.
26. Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: analysis of 600 000 surgeries. Ophthalmology. 2017;124:768‐775.
27. Haripriya A, Chang DF, Ravindaran RD. Endophthalmitis reduction with intracameral moxifloxacin in eyes with and without surgical complications: results from 2 million consecutive cataract surgeries. J Cataract Refract Surg. 2019;45(9):1226-1233.
28. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123:287-294.
29. Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011;37:2105-2114.
30. Shorstein NH, Gardner S. Injection volume and intracameral moxifloxacin dose. J Cataract Refract Surg. 2019;45(10):1498-1502.
31. Arshinoff SA, Modabber M. Injection volume and intracameral moxifloxacin dose. J Cataract Refract Surg. 2020;46(1):162-163.
32. Melega MV, Alves M, Lira RPC, et al. Safety and efficacy of intracameral moxifloxacin for prevention of post-cataract endophthalmitis: randomized controlled clinical trial. J Cataract Refract Surg. 2019;45:343-350.
33. Matsuura K. Long-term effects of intracameral moxifloxacin. Graefes Arch Clin Exp Ophthalmol. 2015;253:2335-2336.
34. Amireskandari A, Bean A, Mauger T. Toxic anterior segment syndrome with intracameral moxifloxacin: case report and review of thel. Case Rep Ophthalmol Med. 2021;2021: 5526097.
35. FDA alerts health care professionals of risks associated with intraocular use of compounded moxifloxacin. US Food and Drug Administration. August 12, 2020. Accessed August 23, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-alerts-health-care-professionals-risks-associated-intraocular-use-compounded-moxifloxacin
36.Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A; ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41(6):1300-1305.