


Vitreous is an extended extracellular matrix that fills the center of the eye with a clear gel (Figure). Despite being the largest structure in the eye, vitreous has long been the least understood of all ocular components.1 Recent studies in both basic and clinical sciences, however, have shed light on the role of vitreous in ocular physiology and pathology, involving both the anterior and posterior segments of the eye.2,3 Consequently, there have been recent paradigm shifts in not only diagnostic methodologies4 but also therapeutic5 approaches to vitreous.
Although the expansion of vitrectomy indications to include vitreous floaters has gained some traction in the posterior segment,5-7 there is limited recognition among anterior segment specialists regarding the role of vitreous in vision and visual quality of life. There is even less appreciation for how vitrectomy can be employed to improve contrast sensitivity (CS) following refractive surgery rather than merely to treat disease.

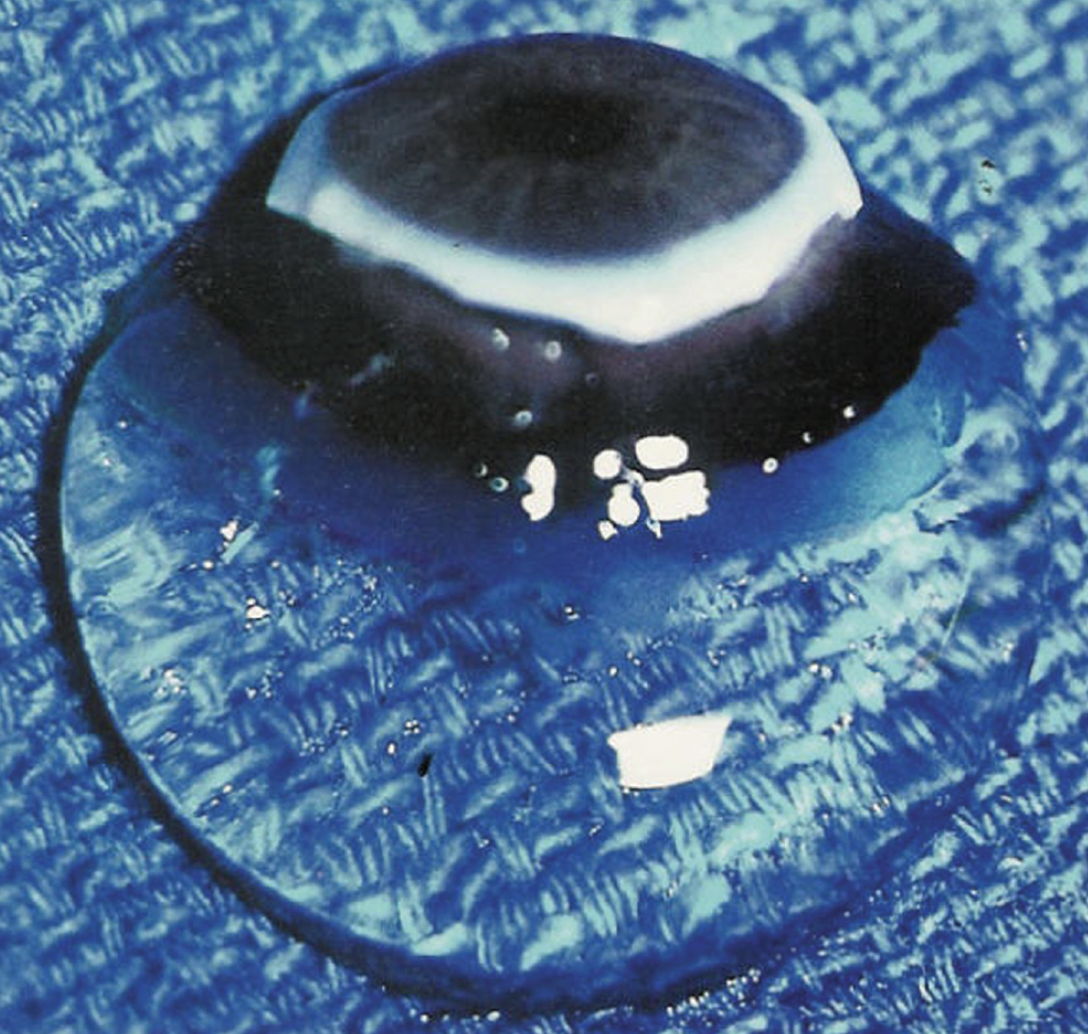
Figure. The vitreous body of a 9-month-old girl was dissected free of the sclera, choroid, and retina. Despite being 98% water and placed on a surgical towel in room air, the gel structure was maintained owing to the supramolecular network of collagen and hyaluronan.
Reprinted with permission from Sebag J. Vitreous—in Health & Disease. New York, NY: Springer; 2014.
MYTH NO. 1: VITREOUS FLOATERS ARE NOT A DISEASE
Vitreous floaters are visual phenomena that are a common complaint. A smartphone survey found that two out of three respondents admitted to having floaters, while one-third reported an impact on their vision.8 Myopic individuals9 and those with posterior vitreous detachment (PVD)10 are most frequently affected. Although practitioners often dismiss floaters as innocuous, many patients feel that floaters have a significant impact on their quality of life.11,12 Studies have determined that patients perceive floaters as comparable to age-related macular degeneration and worse than glaucoma, diabetic retinopathy, mild angina, mild stroke, colon cancer, and asymptomatic AIDS. Notably, patients reported that they would be willing to exchange 1 year for every decade of remaining life to be rid of their floaters.11
Clinicians, however, have struggled to perceive this affliction as serious owing to the lack of objective, quantitative criteria for diagnosis and disease severity assessment. Conventional OCT is inadequate for visualizing the entire vitreous body and is inaccurate in diagnosing PVD.13 Routine clinical assessments, including visual acuity, color vision, and visual field testing, are not affected by vitreous changes. CS, however, is profoundly affected by vitreous opacities. In a study of 140 patients who sought vitrectomy for floaters, there was an average of 91% degradation in CS compared to controls.14 This vision dysfunction has been correlated with visual quality-of-life metrics and the degree of vitreous opacification, as measured by quantitative ultrasonography.12
As a result, Sebag introduced the term vision degrading myodesopsia (VDM) to describe clinically significant cases of vitreous floaters. Stanga, Reinstein, and colleagues in London have advanced the understanding of VDM by developing an advanced diagnostic protocol,15 which includes BCVA, CS function, straylight measurements, infrared confocal scanning laser ophthalmoscopy, and swept-source widefield OCT. These diagnostic tools help evaluate patients with floaters before performing selective vitrectomy, which successfully resolves the problem.16
MYTH NO. 2: THE INDICATIONS FOR VITRECTOMY SURGERY SHOULD BE LIMITED TO RETINAL DISEASES
Historically, refractive surgery focused on correcting or minimizing refractive errors, but it has evolved to include new keratorefractive techniques, such as laser-assisted lenticule extraction, pioneered by Reinstein and colleagues with small-incision lenticule extraction (SMILE) using the VisuMax (Carl Zeiss Meditec).17,18 Future advancements are expected to include options such as presbyopia correction19 and advanced IOLs designed to provide more physiologic correction of refractive errors, as envisioned by Reinstein and colleagues.20 It is now time to expand the indications for vitrectomy surgery to address endogenous and post–refractive surgery degradation of CS.
Any optical surface in the visual pathway can affect vision. Beyond corneal shape and lens opacification, Reinstein, Waring, and others demonstrated that other optical surfaces of the eye can have an impact on quality of vision, such as the air-tear interface21 and early posterior capsular opacification.22 There is a growing body of evidence indicating that vitreous opacities also have optical significance, as demonstrated using advanced methodologies developed by Stanga and colleagues.15 To address these abnormalities, both limited vitrectomy (small-gauge, sutureless vitrectomy that spares the retrolental vitreous and avoids surgical PVD induction)14 and selective vitrectomy (core vitrectomy removing only central vitreous opacities)16 have been shown to treat VDM safely and effectively. Studies indicate that limited vitrectomy significantly reduces vitreous echodensity and normalizes CS.14,23 Stanga and colleagues have employed selective vitrectomy with similar success.16
Given these findings, refractive surgeons should evaluate and discuss vitreous opacities during preoperative assessments, particularly in refractive surgery patients. Surgeons should educate and counsel patients on the potential role of vitrectomy in improving CS by removing vitreous opacities.
MYTH NO. 3: VITRECTOMY IS DANGEROUS
Vitrectomy was developed by Machemer and Parel in the 1970s.24,25 This procedure revolutionized the treatment of posterior segment diseases. Technological advances have since led to the use of smaller instruments and the adoption of a sutureless technique, typically performed under local anesthesia in ambulatory settings. This evolution has expanded the role of vitrectomy to treat VDM from vitreous floaters. Early adopters of this application faced two approaches: one aimed at efficacy with the hope of safety, or one focused on safety with the hope of efficacy. Limited vitrectomy was developed by Sebag with a focus on safety, prioritizing the avoidance of surgical PVD induction to reduce the risk of iatrogenic retinal tears and detachments, and preserving intact retrolental vitreous to mitigate cataract formation.14,23
Studies have shown that surgical PVD induction increases the risk of iatrogenic retinal tears. For instance, not inducing PVD during vitrectomy for macular pucker lowered the incidence of retinal breaks from 32% to 2.1% (P = .006), and in macular hole surgery, retinal tears decreased from 12.7% to 3.1% (P = .008).26 In floater vitrectomy, the incidence of iatrogenic breaks was 30.1% with PVD induction, compared to 11.1% without PVD induction (P = .02).27 Limited vitrectomy, which avoids PVD induction when the posterior vitreous cortex is attached to the retina, was designed to prioritize safety. In a series of 195 limited vitrectomies, postoperative retinal tears occurred in 1.5% of cases, and rhegmatogenous retinal detachment occurred in 1.5%, far lower than the previously reported rate of 6.8% to 10.9%.14
Beebe et al emphasized that the “lens is an unusual structure in an unusual environment” since it has low oxidative metabolism and resides in a low-oxygen environment.28 Vitreous supports this environment owing to its high antioxidant content.29 After PVD30 or vitrectomy,31,32 however, intravitreal oxygen levels rise, contributing to cataract formation.28 Previous studies have reported cataract surgery rates after vitrectomy ranging from 63.2%28 to 80%.33 Most lens opacifications occur within the first 6 months after vitrectomy.34
Limited vitrectomy aims to reduce cataract formation by avoiding surgical PVD induction and leaving 3 to 4 mm of intact vitreous gel behind the crystalline lens. In one series, cataract surgery was needed in only 23.5% of cases at an average of 15 months after vitrectomy.23 Another series reported a 20-month follow-up period with only 18% of cases requiring cataract surgery.35 In the largest series published to date, cataract surgery was performed in 16.9% of cases, with an average follow-up period of 32.6 months.
These data suggest that, in patients with VDM, limited vitrectomy is a safer procedure, offering a significantly lower incidence of both rhegmatogenous events and cataract surgery. Additionally, the approach effectively restores CS, positioning limited vitrectomy as a new paradigm in refractive surgery.36 Stanga and colleagues found similar results with selective vitrectomy.16
MYTH NO. 4: UNHAPPY PATIENTS WITH MULTIFOCAL LENSES CAN BE HELPED ONLY BY AN IOL EXCHANGE
Although many multifocal IOLs offer the benefit of relative spectacle independence, they may have a negative impact on CS. This was a key consideration when patients with multifocal IOLs presented with complaints of floaters. Although these patients were found to have increased vitreous density on ultrasound, it was unclear whether their abnormal CS would improve following limited vitrectomy. Fortunately, studies have shown that, although CS after vitrectomy in eyes with multifocal IOLs did not reach the same levels in monofocal IOL or phakic eyes, there was still significant improvement in CS across all subgroups, including those with multifocal IOLs.37
Additionally, newer generations of presbyopia-correcting IOLs have significantly improved CS and now feature updated International Standards Organization classifications beyond those of multifocal IOLs, such as extended range of vision and full vision range IOLs. Patients seeking better-quality vision after cataract surgery with presbyopia-correcting IOLs should therefore undergo diagnostic ultrasonography and CS measurement. If abnormalities are detected, these patients may benefit from limited or selective vitrectomy.
CONCLUSION
VDM caused by vitreous floaters is a condition characterized by reduced CS, which has a significant impact on quality of life. VDM is safely treatable with vitrectomy (either limited or selective), which can improve CS, even in patients with presbyopia-correcting IOLs.
Refractive surgeons should consider referring patients for vitrectomy surgery if they experience symptomatic degradation of CS following refractive procedures.
1. Sebag J. Vitreous: the resplendent enigma. Br J Ophthalmol. 2009;93(8):989-991.
2. Sebag J. The Vitreous: Structure, Function, and Pathobiology. Springer; 1989.
3. Sebag J, ed. Vitreous—In Health and Disease. New York, NY: Springer; 2014.
4. Sebag J, Sadun AA, Pierce EA. Paradigm shifts in ophthalmic diagnostics. Trans Am Ophthalmol Soc. 2016;114:WP1.
5. Huang LC, Yee KMP, Wa CA, Nguyen JN, Sadun AA, Sebag J. Vitreous floaters and vision: current concepts and management paradigms. In: Sebag J, ed. Vitreous—In Health and Disease. Springer; 2014:771-788.
6. Gui W, Silverman RH, Sebag J. Etiology and diagnosis of vision degrading myodesopsia. Retinal Physician. 2022;19:30-33. retinalphysician.com/issues/2022/june-2022/etiology-and-diagnosis-of-vision-degrading-myodeso
7. Sebag J. Managing vitreous floaters. Retina Today. October 2023. Accessed September 17, 2024. retinatoday.com/articles/2023-oct/managing-vitreous-floaters
8. Wagle AM, Lim WY, Yap TP, Neelam K, Au Eong KG. Utility values associated with vitreous floaters. Am J Ophthalmol. 2011;152(1):60-65.e1.
9. Nguyen JH, Nguyen-Cuu J, Mamou J, Routledge B, Yee KMP, Sebag J. Vitreous structure and visual function in myopic vitreopathy causing vision-degrading myodesopsia. Am J Ophthalmol. 2021;224:246-253.
10. Garcia GA, Khoshnevis M, Yee KMP, Nguyen-Cuu J, Nguyen JH, Sebag J. Degradation of contrast sensitivity function following posterior vitreous detachment. Am J Ophthalmol. 2016;172:7-12.
11. Sebag J. Floaters and the quality of life. Am J Ophthalmol. 2011;152(1):3-4.e1.
12. Mamou J, Wa CA, Yee KM, et al. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Invest Ophthalmol Vis Sci. 2015;56(3):1611-1617.
13. Hwang ES, Kraker JA, Griffin KJ, Sebag J, Weinberg DV, Kim JE. Accuracy of spectral domain OCT of the macula for detection of complete posterior vitreous detachment. Ophthalmol Retina. 2020;4(2):148-153.
14. Sebag J, Yee KMP, Nguyen JH, Nguyen-Cuu J. Long-term safety and efficacy of vitrectomy for vision degrading myodesopsia from vitreous floaters. Ophthalmol Retina. 2018;2(9):881-887.
15. Stanga PE, Valentin J, Reinstein UI, et al. New diagnostic technology and methodology for the assessment of the vitreous, its floaters and opacities, and their effect on vision: standardised kinetic anatomical and functional testing of VFO (vitreous floaters and opacities) (SK VFO Test). Invest Ophthalmol Vis Sci. 2023;64(8):5333.
16. Stanga SEF, Saladino A, Reinstein U, et al. SK-VFO test and widefield swept-source OCT-guided selective vitrectomy (SV) for vitreous floaters and opacities (VFO). Invest Ophthalmol Vis Sci. 2024;65(7):4023.
17. Reinstein DZ, Archer TJ, Gobbe M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye Vis (Lond). 2014;1:3.
18. Reinstein DZ, Archer TJ, Carp G. The Surgeon’s Guide to SMILE: Small Incision Lenticule Extraction. 1st ed. Routledge; 2018.
19. Reinstein DZ, Carp GI, Archer TJ, Gobbe M. LASIK for presbyopia correction in emmetropic patients using aspheric ablation profiles and a micro-monovision protocol with the Carl Zeiss Meditec MEL 80 and VisuMax. J Refract Surg. 2012;28(8):531-541.
20. Ang M, Gatinel D, Reinstein DZ, Mertens E, Alió Del Barrio JL, Alió JL. Refractive surgery beyond 2020. Eye (Lond). 2021;35(2):362-382.
21. Gouvea L, Waring GO 4th, Brundrett A, Crouse M, Rocha KM. Objective assessment of optical quality in dry eye disease using a double-pass imaging system. Clin Ophthalmol. 2019;13:1991-1996.
22. McMillin JC, Rocha KM, Barnwell EL, Haddad JS, Waring GO 4th. Objective evaluation of vision quality in pseudophakic patients with posterior capsular opacification using double-pass retinal imaging. Arq Bras Oftalmol. 2019;82(3):189-194.
23. Sebag J, Yee KM, Wa CA, Huang LC, Sadun AA. Vitrectomy for floaters: prospective efficacy analyses and retrospective safety profile. Retina. 2014;34(6):1062-1068.
24. Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813-820.
25. Parel JM. The history of vitrectomy instrumentation. In: Sebag J, ed. Vitreous—In Health and Disease. Springer; 2014:665-692.
26. Chung SE, Kim KH, Kang SW. Retinal breaks associated with the induction of posterior vitreous detachment. Am J Ophthalmol. 2009;147(6):1012-1016.
27. Tan HS, Mura M, Lesnik Oberstein SY, Bijl HM. Safety of vitrectomy for floaters. Am J Ophthalmol. 2011;151(6):995-998.
28. Beebe DC, Holekamp NM, Siegfried C, Shui YB. Vitreoretinal influences on lens function and cataract. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1293-1300.
29. Ankamah E, Sebag J, Ng E, Nolan JM. Vitreous antioxidants, degeneration, and vitreo-retinopathy. Antioxidants (Basel). 2019;9(1):7.
30. Holekamp NM. The vitreous gel: more than meets the eye. Am J Ophthalmol. 2010;149(1):32-36.
31.Stefánsson E. Vitreous physiology. In: Sebag J, ed. Vitreous—In Health and Disease. Springer; 2014:437-457
32. Jackson TL, Nicod E, Angelis A, et al. Pars plana vitrectomy for vitreomacular traction syndrome: a systematic review and metaanalysis of safety and efficacy. Retina. 2013;33(10):2012-2017.
33. Cherfan GM, Michels RG, de Bustros S, Enger C, Glaser BM. Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am J Ophthalmol. 1991;111(4):434-438.
34. Feng H, Adelman RA. Cataract formation following vitreoretinal procedures. Clin Ophthalmol. 2014;8:1957-1965.
35. Yee KMP, Tan S, Lesnik Oberstein SY, et al. Incidence of cataract surgery after vitrectomy for vitreous opacities. Ophthalmol Retina. 2017;1(2):154-157.
36. Sebag J, Boneva S, Nguyen J. Improving contrast sensitivity with vitrectomy—a new refractive surgery paradigm. Paper presented at: ASCRS Annual Meeting; May 6, 2023; San Diego, CA.
37. Nguyen JH, Yee KMP, Nguyen-Cuu J, Mamou J, Sebag J. Vitrectomy improves contrast sensitivity in multifocal pseudophakia with vision degrading myodesopsia. Am J Ophthalmol. 2022;244:196-204.




