CRST: In your experience, why is the relationship between industry and clinician/surgeon so important to innovation?
Iqbal Ike K. Ahmed, MD, FRCSC: Innovation is a collaborative experience. It is about bringing in ideas from multiple perspectives and experiences to provide the best improvement in or change to current practice.
Michael Amon, FEBO: The cooperation of scientists, clinicians, and surgeons with industry is mandatory for progress. Doctors know best the needs of their patients, and eye surgeons know best how ophthalmic instruments and implants can be improved. Industry has the tools to develop, produce, and bring innovative products to market.
Robert Edward Ang, MD: This feedback loop brings about technological breakthroughs. Both sides contribute to the formulation of ideas—industry provides the resources in product development and clinicians prove safety and effectiveness. Collaboration can lead to the development of products that improve patients’ quality of life.
John F. Doane, MD, FACS: Without innovators coming up with new techniques and technology, humanity cannot move forward. In a modern society with regulations and licensure requirements, innovators cannot treat patients, and few surgeons and physicians come up with innovative concepts and technology. There must be a marriage of the two—innovator and clinician—plus corporate research structure to move clinical research forward in hopes of commercialization and eventual benefits for patients.
Eric D. Donnenfeld, MD: Clinicians conceive ideas on unmet needs and how to improve patient care, but we need the help of industry to take these ideas from the earliest stages to FDA approval. There are few disruptive changes in ophthalmology; most are incremental improvements. We are usually brought into the innovation process by industry to improve upon existing technologies and incrementally enhance how we care for patients.
Marjan Farid, MD: It can be hard for industry to know if a technology or pharmaceutical innovation meets the needs of patients in a clinical setting. The clinical knowledge that physicians have in this area gives industry the spark it needs to drive innovation forward.
Ivan Gabrić, MD: R&D people usually have great ideas. Most of the time, however, they need clinicians’ help in determining how to apply the technology to a human being. Then, the marketing team works to advertise the product and make it popular. Clinicians can also help with this task by sharing their clinical experience with colleagues.
Karsten Klabe, MD: Ophthalmology is a complex, high-technology medical discipline. We use state-of-the-art technology to facilitate both diagnosis and treatment. That is why the relationship with industry is so important.
Guy Kleinmann, MD: We recognize the unmet needs in disease management and patient care and have an idea of what requires improvement. Industry knows how to achieve the change. Either we shape industry’s ideas, or industry helps develop ours. Innovation requires the strength of both parties.
Ben LaHood, MD, MBChB(dist), PGDipOphth(dist), PhD, FRANZCO: Both parties can function well and innovate on their own, but they can achieve far more together. Industry and clinicians bring different skills and resources to the table. It can be powerful to collaborate with parties that come from different backgrounds, have unique ways of thinking, and bring experiences that focus on a specific task. Innovation can produce gradual, incremental improvement or a breakthrough. In either scenario, progress may come from looking at problems from different angles.
The flipside is that professionals from within the industry and clinicians can keep each other in check. Not all innovations are wise or necessary. The character Ian Malcolm in the movie Jurassic Park makes the following comment on bringing dinosaurs back from extinction: “Your scientists were so preoccupied with whether or not they could, they didn’t stop to think if they should.” This cinematic moment often comes to my mind when I preview products in development. Not every idea can or should come to market, and the industry-clinician relationship is important to directing limited resources.
Kaweh Mansouri, MD, MPH: Collaborating with industry allows clinicians to improve approaches to diagnosis and management of patients. It permits us to be actively involved in innovation, to shape it, and to identify areas or needs. The industry point of view may be more financially driven, whereas our point of view tends to center on the patients themselves.
Cathleen M. McCabe, MD: The more I collaborate with industry, the more I understand its importance. Both parties—industry and surgeons—continue to learn how important it is to keep the end user in mind during the early stages of innovation. When new products are developed and engineered without the early involvement of surgeons and others who will interact with the product, we invariably find things that could have been engineered more intuitively to simplify the procedure and make it more effective.
Additionally, we surgeons can help industry pinpoint techniques and tools we desire to have and disease processes we wish we could diagnose earlier or treat more effectively. We may have thoughts on how this might be done, but without industry collaboration, we often can’t take the idea through the R&D process to get it into physicians’ hands.
Jodhbir S. Mehta, BSc(Hons), MBBS, PhD, FRCOphth, FRCS(Ed), FAMS: It’s a truly synergistic relationship—we need each other to drive innovation. We clinicians want to improve patient outcomes, but doing so can be challenging without the help of industry. A lot of great ideas never become a part of patient care. On the other side, it is important for industry to engage with clinicians to find out how to help make improvements—whether it is imaging systems, laser platforms, or diagnostic devices. Understanding the clinical needs of patients can help industry focus its work and drive innovation.
T. Hunter Newsom, MD: The biggest thing is that industry has financial backing and a knowledge of how to work with the FDA and institutional review boards, whereas surgeons use the technologies in development and provide feedback and guidance. We both need each other to keep pushing innovation forward for the benefit of patients everywhere.
Constance Okeke, MD, MSCE: A great deal of innovation is spurred by problems that require solutions. We clinicians and surgeons can identify the obstacles to patient care we experience. We have keen insight into unmet needs and where we need help. Industry has a huge roster of talented people in engineering, manufacturing, marketing, and business and the financial backing to take an idea through research and development and into the marketplace. When industry and physicians collaborate, they can develop innovative solutions that change the field for the benefit of patients.
Shamil S. Patel, MD, MBA: As demographics evolve, I expect certain fields in medicine such as glaucoma will see substantial growth. With that will come unique opportunities. Our industry partners have the infrastructure and capital resources to take a clinical idea from research to development more efficiently than we clinicians can.
The capital investment focused on glaucoma innovation has led to the rapid development of surgical techniques, including the proliferation of MIGS and now minimally invasive conjunctival surgery. Industry uses these advances to compete for market attention, which can lead to safer and earlier disease intervention.
The challenge we face is to ensure a greater depth of development rather than a breadth of options. This depth should include improved diagnostics for IOP measurement and disease progression, a longer duration of action for medications, and the development of surgical interventions that minimize risk while lowering IOP to a similar range as filtration procedures. I believe this is something that we can achieve with the help of our industry partners.
Eric D. Rosenberg, DO, MScEng: Early on in medicine, physicians used to be everything—the doctor, the innovator, and the engineer. They had the best understanding of what was needed to advance the field. As subspecialties developed over time, it became difficult or nearly impossible for a lone physician to have the necessary expertise within a diverse set of fields to produce the same rate of change. Now, large meaningful changes and advances typically require collaboration between physicians, scientists, industry, and engineers.
Leonard K. Seibold, MD: I view the relationship between industry and surgeon as a back-and-forth interaction. Patient care starts with the patient-physician relationship. Industry needs to hear about the struggles with and limitations of current diagnostic and therapeutic options. This helps focus their efforts on developing novel solutions. Physicians depend on innovations to deliver safer, more effective patient care. Ongoing collaboration is vital to fine-tuning developments and recognizing and addressing their limitations.
Oluwatosin Smith, MD: We clinicians are the ones who see patients, so we recognize the current and future needs in patient care. We are not, however, the ones to carry innovative ideas all the way through to a finished, marketable product. That is industry’s role. We need industry, and industry needs us. That is the way I see it.
Karl G. Stonecipher, MD: If industry and physicians cannot work together, then innovation will be slowed by delay after delay at the FDA. I am not bashing the agency, but some of its long-standing employees, including the longtime director of the FDA’s Division of Ophthalmic and Ear, Nose & Throat Devices, Malvina Eydelman, MD, have recently departed for other jobs. Dealing with new contacts at the FDA complicates the approval process.
Collaboration between industry and physicians is more important now than ever before. It generally produces superior study protocols, which can allow the clinical trial process to move more smoothly and rapidly.
Kevin L. Waltz, OD, MD: I am currently working on a beta version of a femtosecond laser for glaucoma. An alpha version was invented by some very bright PhDs but without input from surgeons. Fellow surgeons and I helped design the beta version that can better interface with patients. I often tell industry members that, if a technology is for use in surgery, then a surgeon must be invited to sit at the table. Surgeons have a different perspective on patient care. For example, industry makes advanced IOLs but does not implant them or provide postoperative management to the patients who receive the IOLs. With a few exceptions, surgeons do not make IOLs. When it works well, the partnership between industry and surgeons expedites the innovation process, so the world at large gets better products faster.
CRST: What does the clinician/surgeon bring to the table to help bring meaningful innovation to market?
Dr. Ahmed: Industry’s perspective on innovation is to bring a benefit to society and to be profitable in the process. Our perspective as physicians is to provide meaningful improvements to the quality of patient care. Some of the best consultants are also able to look at the financial piece and understand what drives innovation. Most important, however, is that we clinicians and surgeons serve as advocates for our patients. We are looking for ways to improve their quality of life—be it by preserving vision, making postoperative management easier, providing more long-lasting and effective treatment, facilitating adherence to and persistence with prescribed therapy, or offering a lifestyle benefit.
Most innovations, moreover, require our insights to be successful. We are the ones to use the technology and can thus convey the parameters of adoption. For example, how easy or difficult will it be to incorporate into practice? How disruptive will it be? What are the barriers to adoption, and how should surgeons be trained?
Professor Amon: During their medical education and careers, clinicians and surgeons develop a deep knowledge of their specialty. Eye surgeons perform thousands of surgical procedures and understand how instruments and implants should perform inside the eye. If these physicians realize how to improve outcomes and if they develop ideas on how to overcome surgical difficulties and improve patient satisfaction, they are the right people to help industry develop implants and devices. During the process of cooperation, options often evolve, and collaboration can both optimize results and lead to new frontiers. The knowledge and understanding of all involved parties improve, the doctor comes to recognize the potential and limitations of industry, and industry benefits from the innovative and creative power of the surgeon.
Dr. Ang: We clinicians have direct contact with the end beneficiary—the patient. We have a direct line to what they need, want, and like, and we ourselves are often the customer (ie, the ones who use the technology). All products must be tested and validated. Our role is to provide feedback on whether a product requires improvement, is good enough to be released, or should not make it to market.
Dr. Doane: We bring faith that the device or technology can deliver the intended effect(s). Clinicians and surgeons are willing to step beyond their comfort zone despite knowing that what they are embarking on may not work or could be harmful.
Some clinical research is a dead end, and I feel for the innovators whose efforts come up short. I have worked on projects that spanned 5 to 15 years from genesis through my involvement. With some research that lasted more than 10 years, the technology simply did not pan out despite the time and effort invested. Even if a product tests well and gets approved, however, it can still be a commercial failure. This is another pitfall. Hard work pays off, and many commercial successes occur. More importantly, however, the treatment improves patient health and well-being. Those are rewarding outcomes for all involved.
Dr. Donnenfeld: We surgeons bring clinical experience and expertise that industry doesn’t possess. Our contribution to the partnership with industry is our ability to recognize unmet needs in patient care. We also provide an objective, evidence-based approach to evaluating ideas that many times shapes the way industry develops a product. Sometimes, our feedback helps a company decide that a certain innovation is not worth developing or should be approached from a different perspective.
Dr. Farid: Physicians can pinpoint specific clinical needs when we are operating. We focus on safety, find gaps clinically, and sometimes have ideas that might improve clinical and surgical efficiency. We are always thinking about technologies that could enhance patient care and improve patient outcomes and satisfaction. We have our fingers on the pulse of what our patients need and what current technology lacks, and we can share that with industry partners at the forefront of bringing these technologies to market.
Dr. Gabrić: Each product has a life cycle. Early in that cycle, it becomes apparent whether the product is viable. That is when a small initial medical advisory board helps shape the product. Afterward, when the product is used in the first few patients, early-stage clinicians can influence the experiences of future patients and clinicians. In other words, the advisory board ensures that the device is not creating a problem and that it is useful, but then volume becomes necessary. Clinicians and surgeons are needed to use the product or perform the procedure. They ask questions. Can you change this button? Can you add X function? Can you do Y because I have a patient who needs it? The early user experience is what really shapes a product.
Everyone sees revolutionary products come to market. Not widely visible are the 15 surgeons who were the early users and helped shape the technology by working directly with the manufacturer and the R&D team to make changes. When you are asked to be a part of a project at an early stage, you have an opportunity to influence not just the company’s and product’s future but also all the procedures to be performed with it and their outcomes.
Without the clinicians using the equipment, what is the point of its existence? It could be the best laser system, phaco machine, or OCT device on the planet, but what does it matter if no one uses it?
Dr. Klabe: We physicians recognize diagnostic, therapeutic, and surgical challenges first and may have ideas about how to overcome a problem. We can help industry develop a device or pharmaceutical solution. For example, clinicians suggested the potential role of antivascular endothelial growth factor therapy for the treatment of retinal disease. The next step was industry’s creation of a specific molecule for intravitreal use
Dr. Kleinmann: We shape industry’s ideas for the benefit of patients. We have a sense of whether a proposed concept could be meaningful to patient care because we are the ones in the clinic and the OR.
Dr. LaHood: I have been blown away by the massive, expensive, and prolonged process of innovation. Ideas must be considered in terms of resources, commercial need, fashion, market forces, production capabilities, clinical trials, and regulatory requirements. Clinicians can draw on their real-world experience to reflect on whether patients would want or benefit from a product in development, potentially allowing industry to abandon a project early on in favor of something more promising.
Clinicians can also give their seal of approval to an innovative product or procedure and thereby encourage their colleagues to try it. Most ophthalmologists are creatures of habit—and for good reason. The rate of success with many ophthalmic procedures is close to 100%, but complications can be devastating. Initial trials of innovative devices and techniques—often funded by industry to achieve regulatory approval—are viewed with skepticism by many ophthalmologists. Real-world data collected and published by colleagues carry weight. Clinicians who test and report on innovations can be extremely helpful to their peers and industry by providing the evidence for or against making a change.
Dr. Mansouri: Companies often lack a clinician’s understanding and experience. Many innovations are initiated by PhDs in engineering, biologics, and the neurosciences. Working with clinicians early in the process helps them to elucidate the need and determine how to proceed, including designing the product and studies, conducting clinical trials, and identifying with whom to collaborate. In my experience, when startups partner with experienced clinicians early on, the pathway to clinical approval is shorter.
Dr. McCabe: Surgeons help define the need from the perspective of the end user. Then, once a prototype is ready for testing or a product is on the market, our role is to give feedback on how it can be made better. It’s rare for the first iteration of an innovative technology to be the final version. Innovations are improved by closing the feedback loop between the developer and the user.
Dr. Mehta: We identify gaps in knowledge and patient care that can be addressed by innovation. Clinicians are also in the unique position of being able to test new innovations and, importantly, provide honest feedback.
Dr. Newsom: After 22 years of participating in clinical trials of technologies and procedures—only some of which worked or made it to the market—I have a sense of which ideas are good and which are bad. Sometimes, what is being proposed is improving on an old idea that worked. Other times, an idea is being rehashed that did not work in the past and probably will not work in the future. In these situations, physicians can help move innovation forward by identifying unmet needs and brainstorming various approaches to filling the gaps in patient care.
Dr. Okeke: We bring real-world experience to the table. We are also able to articulate why something does or does not work well, and we can identify concepts that are redundant. Based on our feedback, a company can make changes to a product or procedure or scrap it to devote resources to something else. Further, we can often spot trends and share insight into where the field is going so that industry can respond. Another thing we bring to the table is investigator-initiated research, which provides real-world knowledge that can help our peers make better decisions on how best to use certain products or tools (Figure 1).
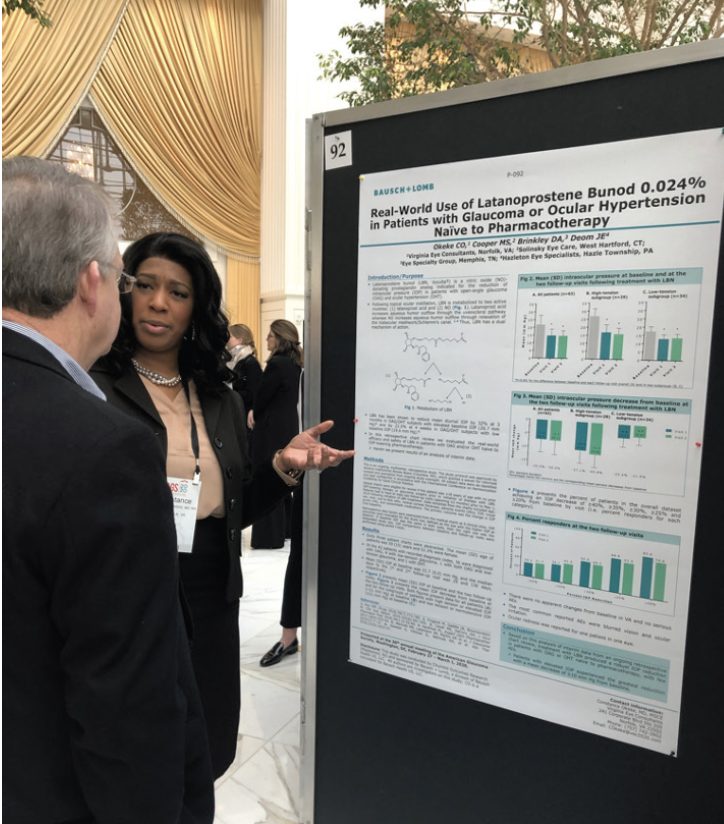
Figure 1. Dr. Okeke presenting investigator-initiated research results on Vzyulta (Bausch + Lomb).
Courtesy of Constance Okeke, MD, MSCE
Dr. Patel: The surgeon’s clinical and intellectual contributions are often seen as the most valuable aspect of the partnership. I, however, believe our biggest contribution is the preservation and development of the physician-patient relationship. Our oath is to patients first. We are vested in them and have their best interests in mind, including the development of earlier, safer, and more effective treatments for glaucoma.
When physicians are included in the development of patient applications and technologies, it helps ensure that industry keeps the patient as the central focus. As partnerships develop, different interests (ie, shareholders) are included in the partnership process, and our duty is to maintain the focus of development on our patients primarily.
Dr. Rosenberg: We clinicians and surgeons are the ones who implement technologies in everyday practice. We are the ones who can provide meaningful and useful feedback to industry members, scientists, and engineers so that technologies can be developed and improved, thereby returning even better products for use with and in our patients.
Dr. Seibold: Clinicians/surgeons bring their frustrations and those of their patients to the table to illustrate unmet needs in medicine. Examples include diseases for which no effective therapy exists, medications with intolerable side effects or poor efficacy, insufficiently effective surgical methods and procedures associated with sight-threatening complications, and diagnostic imaging that is prone to error and time-consuming.
Dr. Smith: We bring expertise in the form of clinical experience, a basic knowledge of disease entities, and recognition of the gaps in patient care. Of these, identifying the gaps is especially important because we provide industry with the insight needed to overcome common unmet needs. What you discuss or the work you do with one company should be separate from what you do with another. It is important to maintain confidentiality and provide honest opinions when asked.
Dr. Stonecipher: We are aware of something that went wrong in the past. Maybe we missed a contraindication for an IOL during the preoperative evaluation, maybe we encountered problems with the haptics of a toric IOL, or maybe we recognize that a proposed product does not offer benefits for clinical or surgical care.
Dr. Waltz: Venture capitalist William J. Link, PhD, is famous for saying the following: “If you fail, fail early.” Industry needs surgeons who have the knowledge and self-assurance to kindly tell a PhD team, “No, your innovative product or procedure does not work the way you want it to,” or “It should be done another way.” Otherwise, limited resources are wasted. The right surgeon can envision what a technology in development can become. That person can help determine if spending millions of dollars could improve the technology or if it is ready for market. The surgeon is a gatekeeper.
Surgeons can also analyze and contextualize data. I am actively involved in research in Central America. Almost all patients who receive a presbyopia-correcting IOL are happy because they had dense cataracts preoperatively. The surgeon’s job is to be cognizant of the big picture, not just the raw data.
CRST: What were some of your earliest experiences with industry, and how have those experiences changed/grown over the years of collaboration?
Dr. Ahmed: Some of my earliest experiences were sitting in on or speaking about a particular product or technology at industry meetings and interacting with representatives who visited my office—typical first experiences for clinicians and surgeons. For me, that evolved into consulting and serving as a primary investigator or medical monitor for clinical trials. Later, I became involved in the research and development of early-stage devices and pharmaceuticals, preclinical work, and even idea creation at startup companies.
Fairly recently, I changed my schedule to allocate more hours and days to my work with industry. I still prioritize clinical care, but now I devote 10% to 20% of my time to nonclinical activities, including collaborating with industry.
Professor Amon: At the start of my first project, it was not easy to find the right members of industry to talk to, but my overall experience—especially with R&D—was positive. I think, if your idea is original and has potential, you have a chance to convince a company to explore your idea and, in the best-case scenario, transform it into reality. One of the main obstacles is regulations. In Europe, where I practice, regulations recently became even more restrictive. Developing a device can be significantly more time-consuming and costly now. Regulatory rules are a necessity, but the Medical Device Regulation adopted in 2017 and others can hinder progress to some extent.
Dr. Ang: I started out as a clinician treating patients. Then I began to discuss my experiences and how to improve outcomes by giving presentations at major meetings and writing articles. I guess industry noticed because I was invited to share my feedback on technology and, eventually, help develop new products.
Dr. Doane: I was fortunate to connect with some amazing innovators and folks who can move innovative ideas through the clinical regulatory and commercialization processes. We keep finding new projects to collaborate on. They know me, and I know them. We have become like a lock and a key.
Dr. Donnenfeld: Most of the early collaborative work I did was evaluating patient care through clinical studies and publishing and presenting my findings. The structure and support of a partner in industry allowed me to run clinical trials that I couldn’t possibly have done on my own and to work hand in hand with industry to develop new ideas and change patient care. I have found this to be extraordinarily rewarding.
My first experience working with industry was in 1989, when I was invited to participate in the FDA clinical trials of the Visx excimer laser (now Johnson & Johnson Vision). I spent half a day every week at the Manhattan Eye, Ear, and Throat Hospital studying excimer laser photoablation. After several years, the laser was approved by the FDA. That experience of devoting 5 years to working—unpaid—with an industry partner positioned me to understand the importance of collaborating with industry and allowed me to have a significant impact on refractive surgery, which was a fledgling specialty at the time.
Dr. Farid: I got involved with industry early in my career mostly because of my mentor, Roger F. Steinert, MD. He understood the crucial link between industry and clinicians. He was at the forefront of technological innovations, applying technologies to gap areas in ophthalmology, and advancing surgical techniques, including corneal transplantation and cataract surgery.
During the past 15 years, I’ve been involved with a lot of technological advances that offer patients a much better quality of vision and more options for spectacle independence, even those who have undergone corneal transplantation. In my opinion, this is largely due to the increased collaboration between surgeons and industry.
Dr. Gabrić: One of my first experiences was working with Advanced Medical Optics on the first generation of multifocal IOLs. The clinic where I practice was one of the study centers in the multicenter study.
IOLs are easy to integrate into your workflow because the surgery is basically the same regardless of the implant type. You monitor patients and track and critically evaluate the results. IOL research can be a stepping stone to more complex research such as software changes to a laser platform and testing other devices, particularly for elective procedures such as refractive surgery. Any loss of BCVA can degrade their quality of life, so it is important to detect and respond to slight changes in the machine and patient outcomes quickly.
There must be a lot of trust in partnerships. If the company culture prioritizes making something to sell it, it may not be in your or your patients’ best interest to adopt it. The manufacturer, meanwhile, must have confidence that your and their interests align. The goal should be to provide the best possible care to patients. People often think that manufacturers want yes people. In my experience, the R&D team and company leaders want doctors to provide honest feedback quickly because it is not possible to hide that a product is a dud. It is in their best interest to make their products as good as possible before they hit the market.
Dr. Klabe: Early in my career, I was proud to provide input on industry’s potential advances and new products, but my feedback was largely limited to yes or no (ie, it does or does not work as expected). Today, I engage in intense discussions with industry colleagues and collaborate with them on developing solutions. The interactions continue to be a learning process, but I have become better at presenting my ideas and understanding what engineers tell me.
Dr. Kleinmann: Early on, I was presented with some innovative ideas that proved to be impractical. As I gained clinical experience and interacted with industry more, I learned how to provide more meaningful feedback to companies, taking an impractical idea and making it practical. I also have a better sense of which ideas are unlikely to pan out and which could with some work because I better understand the obstacles to innovation, the money required to develop them, and the market needs.
Dr. LaHood: I knew little about industry during my training and conducted my research independently. I enjoyed discussing projects with industry colleagues, but I never engaged in a formal relationship. When I attended my first advisory board meeting, I asked, “What is a KOL?” I was told with a smile, “You are a KOL, a key opinion leader.” I cannot change my past lack of exposure, but I strive to make a difference for current trainees. I organize an advanced trainees day at the Australian Society of Cataract and Refractive Surgery where participants learn about topics that are not typically covered in training programs such as mental preparations for operating. This year, I plan to focus on working with industry.
My early experiences with industry mainly involved national-level discussions about conference presentations and messaging that assistance was available to me. The interactions developed into invitations to trial new products and present my results at conferences in the Asia-Pacific region. In recent years, I have been asked for advice at an earlier stage of product development and received support to discuss my research results around the world.
The information sharing and support I have received through my relationships with industry have been incredibly positive. My career would be different without those opportunities. I like to think that industry, in turn, has benefitted from my experience, advice, and communication with colleagues.
Dr. Mansouri: I was fortunate to start working with industry during residency. Two doctoral students at the Swiss Federal Technology School had an idea for a smart contact lens. We met by chance, shared ideas, and went our separate ways. I met them again 2 to 3 years later after they had their first prototype and were thinking about how to design clinical trials. A few years later, after the device received regulatory approval and became commercially available, they started a company, Sensimed, and hired me as their chief medical officer. That was my first serious experience with industry. I remained on the team for almost 10 years.
The experience showed me how to bring a product to market and the challenges of doing so. I learned that having an innovative, functional product does not guarantee commercial success. Other factors come into play. We had developed a smart contact lens, the Triggerfish. It was safe and efficacious, and it was better at collecting IOP data than Goldmann applanation tonometry. The technology was also expensive, and it was hard to convince health authorities and insurance providers in many countries to offer reimbursement. Additionally, the device did not make clinicians’ lives easier. It produced a lot of data that they had to spend more time interpreting compared to simple tonometry. The product was innovative but not a commercial success.
A few months ago, I became the chief medical officer of iStar Medical, which developed the Miniject, a promising silicone device that is implanted in the suprachoroidal space. It is currently approved in Europe, and FDA trials of the device are ongoing.
Dr. McCabe: One of the earliest ways to get involved with industry is by participating in advisory board discussions. Early in our careers, we are more likely to be invited to consult on products that are already on the market. These early engagements are a great opportunity to develop relationships with and a direct line of communication to industry. After the relationship-building stage, the real collaboration begins. At that stage, there should be more opportunities to help advance ideas and products in earlier phases of development. Sometimes, this requires having hard conversations when you may not agree with how things are being done.
Dr. Mehta: When I was a trainee in the United Kingdom, my fellow trainees and I did not collaborate much with industry because we worried how that would be viewed by our colleagues. We did not want to be industry spokespeople for devices or technology. When I took a faculty position in Singapore, however, I saw the advantage or at least the importance of having some dialogue with industry partners. To this day, I decline to be a spokesperson for companies’ products, but I try to show advantages of certain technologies and guide innovation. I generally work with many industry partners as opposed to a range of products from one industry partner.
My early experience was with Carl Zeiss Meditec. It allowed my colleagues and me to be innovative in 2008 and 2009 with our research into wound healing with femtosecond lasers. That work has developed over time, as has my relationship with the company, which broadened to include other technologies. It has never, however, precluded my working with other members of industry.
Dr. Newsom: Early in my career, I expressed my interest in industry collaboration to the sales representatives I got to know. Those who noted my surgical skills and outcomes and my interest in research were receptive, which allowed me to partner with industry. In the beginning, I was looking to learn and to add to my skill set. Now, 22 years later, members of industry contact me to say they are looking to invest in something and request my opinion.
Dr. Okeke: My work with industry dates back to medical school. I participated in the National Medical Association’s Rabb-Venable Excellence in Research Program for 2 years, during which time I won an award sponsored by Alcon. The experience introduced me to people at the company who were committed to supporting young doctors, researchers, and underrepresented minorities in medicine.
Soon after I began practicing medicine, I discovered MIGS in the form of the Trabectome, which is currently manufactured by MicroSurgical Technology but was owned by NeoMedix at the time. I became a consultant for the latter company. It was a small company and could not offer the support to doctors that a larger company can, but the experience of helping to improve patient care through the development of less invasive surgical options was exciting. I delivered lectures, trained other surgeons, and shared with the company my feedback about the product and ideas on how to get it into the hands of more surgeons (Figure 2). It was an inspiring experience. I even wrote a book called The Building Blocks of Trabectome Surgery. The partnership led to my collaboration with MicroSurgical Technology on both that device and other products.
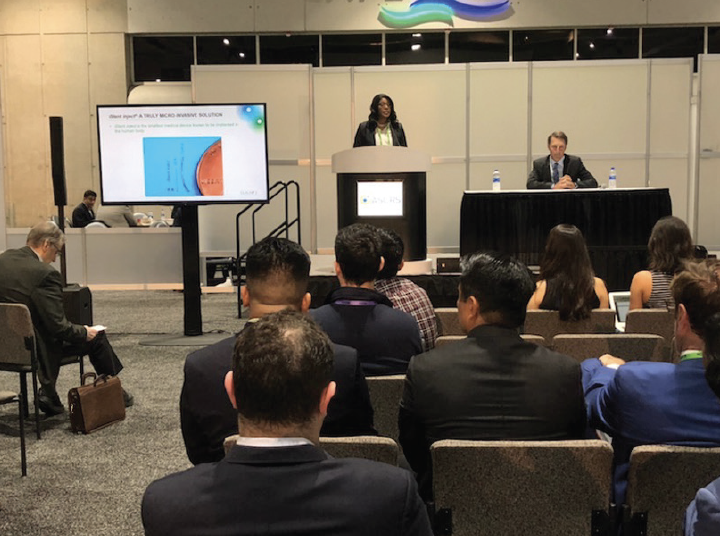
Figure 2. Dr. Okeke lectures on MIGS at an ASCRS Surgical Spotlight.
Courtesy of Constance Okeke, MD, MSCE
My early years consulting led me to work with numerous pharmaceutical companies and device manufacturers on clinical trials. My first participation in a clinical trial was with Glaukos.
Dr. Patel: I’m relatively new to working with industry. I work with a few companies that manufacture some of the surgical glaucoma treatments I offer to patients. Early on, this collaboration provided the additional resources I needed to evaluate my surgical outcomes and refine my techniques. I found this level of involvement to be exciting. It was almost a personal laboratory, where I could evaluate how minor changes in technique affected outcomes. The data were not valuable externally, but they helped me refine my surgical technique.
Over time, my partnership with industry has grown. I teach surgical techniques to other surgeons, which expands patient access to glaucoma technologies and procedures.
I love the discussions and friendships that I have with colleagues and industry partners as a direct result of collaboration. These relationships were unexpected and have been refreshing.
Dr. Rosenberg: In the beginning, I lacked the full picture. The more experience I gathered and the more collaborations I had, the better I understood how much goes into product development. Not every good idea takes off. Success on many different fronts is required to make a product a reality. This is an unfortunate truth.
My work with industry has allowed me to appreciate facets of the innovation process that I did not recognize earlier in my career. I have started to understand the timing of development—what comes first, second, third—and to be able to make recommendations accordingly.
Dr. Seibold: My earliest experiences with industry focused on surgery and MIGS devices that were just coming onto the market. It was an exciting time in glaucoma when industry and physicians seemed to be learning right alongside each other. A collaborative relationship was vital to figuring out how and when to use the technology and procedures.
I continue partnering with industry as new devices come to market, but now the collaboration centers on the nuances of each procedure and where it fits best in the surgical paradigm.
Dr. Smith: I started working with industry about 15 years ago. My first appointment was on a medical advisory board. Board meeting discussions aided the evolution of some of the medications that we currently use in glaucoma.
Since then, my focus has shifted not only in how I interact with industry but also in the things that I collaborate on. I transitioned to discussing current and future developments in MIGS, alternate medication delivery platforms, disease diagnostic tools, and lastly, new and future treatments for glaucoma. We have more in-depth discussions, such as acquisitions—my thoughts on various products and how I think they will work in the market. What do you think about a product? How do you think it will fare in the market? These questions help industry decide on the next step in the process.
Dr. Stonecipher: When I was a chief resident at Tulane, Alcon and Allergan brought several other residents and me on a tour of their campuses. We learned about their research and products. Members of the companies then sat with us individually and asked how they could make our introduction to ophthalmology better. That sort of outreach might have encouraged me to use some of their products, but mainly it formed a positive foundation for future collaboration. Today, I collaborate with industry on an elevated level. I can help companies decide if they want to spend millions of dollars developing a particular product or device. This sometimes requires having difficult conversations, like telling a company what is wrong with a new potential product. It also requires me to suggest minor or major adjustments to great devices that are already available but could be made more efficient or safe.
Dr. Waltz: I got into research in the late 1990s as a favor to a friend in industry, Nick Tarantino, OD, FAAO. I was implanting a lot of Array IOLs (no longer available) at the time. He worked for the manufacturer, Advanced Medical Optics. He asked me to conduct a controlled trial of a new version of the lens. I had no idea what that meant, but I agreed because I like and respect Nick. The trial process was interesting, but what really fueled my fire was that the trial gave me a standard by which to judge myself. Many surgeons do not want or need to compare themselves to their peers. I felt differently. The experience allowed me to improve as a surgeon because it showed me where I could improve.
In 2012, my colleagues and I conducted the first clinical trials in the world of an extended depth of focus IOL (Tecnis Symfony, Johnson & Johnson Vision; Figure 3) in Honduras. We were able to complete the trials in that country much faster than could have been done anywhere else. According to company representatives, this allowed the technology to be brought to the worldwide market approximately 20 months earlier than it would have been otherwise.
Figure 3. The first Symfony IOL implanted in a human eye in September 2012 in Honduras.
Courtesy of Kevin L. Waltz, OD, MD
My colleagues and I have been performing research in Central America ever since because we can complete trials faster there than elsewhere with full government and Institutional Review Board approval. This improves the rate and quality of technology available to patients worldwide while supporting needed care in Central America. Being a part of the process has been really rewarding.
CRST: How do you choose with whom to partner?
Dr. Ahmed: I am generally open-minded. I look at any potential opportunity that someone approaches me with. I feel lucky and grateful that people approach me to collaborate on innovations in our field. It is an honor to be asked to be involved. That said, I have to be excited about and believe in a project to commit to investing my time in its development. That usually means the product or procedure is different and disruptive. I also must feel comfortable working with the people who are behind the partnership. If the first experience is good, I always consider working with the company or individual again.
Professor Amon: For me, the main selection criteria are the quality and history of a manufacturer. The company should have demonstrated superior expertise in its field. It should have years of experience, and its products should be used worldwide. The company should also have demonstrated an interest in publishing its data and results in peer-reviewed journals and should not be driven by marketing aspects alone.
The company must be big enough to have the financial resources to develop new products. I prefer companies, however, that are not too big because it can guarantee personal communication. I also prefer to collaborate with European companies to make the associated travel and legal requirements easier to meet, but this is not mandatory.
Dr. Ang: It starts with my field of expertise and my passions. I am attracted to technology that can help the patients I see regularly in my practice. This also helps industry partners and me to evaluate my recruitment capabilities.
Additionally, I have worked with many industry researchers, and these relationships usually lead to more projects together.
Dr. Doane: It is part product and part people. I favor device work. I am not made for investigating and developing medical therapies. The people I work with are also into devices and not medicine, so we are a match.
Dr. Donnenfeld: I think the best advice that I can give anyone who is interested in working with industry is this: When you have the opportunity to work with a company, take it. In the beginning, it is flattering just to be asked. Over the years, I have become more selective about my partnerships. I choose companies that have innovative ideas that I believe could have a significant impact on the field of ophthalmology and make a difference in patient care.
When I started working with industry, I developed relationships with specific people. I continue to work with these like-minded individuals because I know they have my personal and my patients’ best interests at heart.
Dr. Farid: I partner with companies that have products I believe in and am excited about and that I feel will have an impact on ophthalmic care. When I can really get behind an innovation, whether it is a device, a drug, or a technique, I am invested in helping the company make it better.
Dr. Gabrić: Fifteen years ago, nobody was partnering with the center where I practice because it was a regional player in an uninteresting part of the world, Southeastern Europe. My colleagues and I showed that the data we provide are honest and of high quality, our follow-up is meticulous, and our feedback is truthful. Industry leaders noticed and began offering us opportunities to perform limited series testing and clinical studies.
We partner with companies whose people and products we believe in. If I am going to be an early adopter of a device, I talk to the engineers. If they inspire trust and exhibit passion when explaining their device, it is more likely to be a good fit for me.
Dr. Klabe: I focus on technological, engineering, hardware, and software solutions in the field of glaucoma with a special emphasis on surgery.
Dr. Kleinmann: When I first meet with a company, I want to talk to everyone I can, learn about the product, and try to understand what they need from me. I agree to a partnership thereafter only if the initial meeting sparked my interest, the project is within my area of expertise, and I feel that the work will be meaningful. It is also important to have some chemistry with the people at the company.
Dr. LaHood: The decision seems daunting, but it is actually simple. I partner with companies whose products I use and whose people I trust. I have researched, published on, and discussed the IOLMaster700 (Carl Zeiss Meditec), and I trust the company’s local team in Australia. I have used and studied the Clareon family of toric monofocal IOLs (Alcon) and found the company’s global and local teams to be supportive. I can share my frank opinions about these products with colleagues without discouragement from my industry partners.
Perhaps a better question is how to determine with whom not to partner. I reject invitations to talk about things on which I am not an expert, even if the payment proffered by a company is tempting.
Dr. Mansouri: My approach has changed over the years. I am an active clinician-scientist and surgeon. I cannot accept every invitation I receive, even if I am interested, because my time is limited.
Two criteria have guided my partnership decisions since I began my career. First, I must find the technology or product exciting and innovative. Second, it must have the potential to improve disease management or diagnosis and have a positive impact on patients’ lives.
Two newer criteria are based on my experience. They have to do with the company itself. How well is it structured? Also, are the people with whom I will be working—the management team, R&D team, and/or the marketing group—competent and agreeable to work with, and do they have a vision similar to mine? I was once involved with a company whose board members had a different mindset than mine. They wanted to drive the commercial aspect aggressively before I, as a clinician-scientist and chief medical officer, thought enough clinical trials had been performed to understand how to use and present the technology.
The last criterion is whether I think the company will be a loser or a winner. I have been associated with both, and I hope my experience gives me a better sense today of which companies will succeed. If I decide to work with an entity, I want it to be a winner.
Dr. McCabe: Initially, it feels flattering to be asked your opinion at all, and you may feel like you want to say yes to everyone. I keep three basic principles in mind when deciding whom I partner with. First, I want it to believe in the product. If I am asked for my input because a company assumes I am an expert in that area, I am honest about what I can and cannot offer. Second, I consider confidentiality agreements I have with other companies and am honest about competing relationships from the beginning. Third, I look for a partner that I feel will respect and value my time, communicate well, and not take advantage of my contribution to the specific innovation. We physicians are thrilled to help advance the field of ophthalmology, so a lot of times we’ll jump head first, giving a lot of our time and expertise, without understanding what the relationship will be. It is important to make sure the relationship and the associated compensation are well defined.
Dr. Mehta: If my perspective on technology corresponds with what a company is trying to achieve, then I find partnering together easy. For example, if I find that I can get some utility out of a product and improve patient care with it, then I collaborate with the manufacturer. I never partner with a company that does not share my vision of patient care. Nor do I partner with companies that just want a spokesperson for a product.
Dr. Newsom: When I was a young surgeon, I was willing to collaborate with anyone who wanted to partner with me. I was fortunate to collaborate with a couple of small IOL companies and develop good relationships with people working on those projects. As their careers advanced, they moved on to bigger companies. Based on our positive past collaborations, they invited me to participate in studies with their current companies. Those relationships and the reputation I have built influence my partnership decisions and opportunities to this day.
When considering partnership opportunities, it is important to consider the goal. Some companies look to reinvent a bad wheel. That is not going to accomplish anything. Others have a tool and are seeking a use for it. That is not likely to be a good study. Neither of these scenarios is a project in which I want to participate.
Dr. Okeke: I tend to choose collaborators that value education and honest, critical feedback. I am not going to tell someone that a product is great if I do not feel that way. I typically partner with companies whose products I use, but I have worked with companies whose products I do not like and explained where I felt improvements were needed. If I feel I can bring value to a collaboration, then I will proceed.
Dr. Patel: My choices are natural fits given the surgeries I perform. I ask the following questions when evaluating a partner.
- Is the partner’s primary focus on patients?
- Will the partner provide the administrative and structural support to help physicians innovate over time?
- Has the partner shown a strong commitment to patient care and research?
- What is the partner’s 5- to 10-year plan?
I like to partner with companies that have cultivated a deep and growing interest in the disease and its treatment.
Dr. Rosenberg: Early on, I enjoyed learning from anybody. Now, I seek partners that bring revolutionary products to the market. There is nothing wrong with evolutionary products, but I prefer to focus on those that have the potential to change the field. I want to partner with companies whose products are going to change my patients’ lives for the better.
Dr. Seibold: I want to offer patients the best possible therapies in a timely manner. I choose industry partners that are developing innovations I think have the potential to improve patient care. I prefer companies that emphasize patient-centered innovation but also genuinely care about their employees and the physicians with whom they partner.
Dr. Smith: Initially, you may not have a lot of choice. At my stage of experience, however, one thing I look at is the product. I need to believe in it and be excited about it to agree to a collaboration. How will the product affect patient care? Will it cause a big change? How safe is the device? If I have a concern, a partnership may present an opportunity for discussion. I can recommend a change to make a product safer, more effective or efficient, or easier to use.
After working with a company for many years, a relationship develops, and it becomes easy to talk about potential new projects. Alternatively, a new, less established company may bring exciting ideas to the table, and it is easy to agree to work together.
Dr. Stonecipher: The key part of the question is choose a partner. It is nice to be asked, but there are reasons to pass on some opportunities. One is if you know nothing about the product. If it does not fall within your area of expertise, you are unlikely to be useful. For example, I remember being asked to consult on a glaucoma device. I declined because I do not provide glaucoma care and did not think I could make a meaningful contribution. I find that companies respect decisions like that and often contact me later about a more suitable opportunity for collaboration. I also choose companies I know. When I understand their products, what their general counsel is doing, and what their leadership is doing, I can bring more to the table.
Dr. Waltz: Almost all of my partnerships derive from personal referrals. My organizations do not advertise. When we receive a phone call from a friend in industry who asks me to talk to another crew about an interesting project, I take the call. New partnerships demand personal introductions, and the requests are based on the reputation and trust of the respective teams.
CRST: How do you balance working with multiple companies?
Dr. Ahmed: I am blessed to have consulted with more than 60 companies, ranging from tiny, early-stage organizations with four employees to billion-dollar corporations. The balancing act can be tricky when competition is involved. My first rule is to be open about what I am doing. The second is to establish trust with the companies, honor confidentiality, and provide high value.
Some people in industry do not feel comfortable with somebody who works with many companies, and that is their right. Generally, however, I find that those in industry see the value of a consultant or surgeon who can bring broad experience to the table. Ultimately, it is about character, but it also demands being careful and organized. Additionally, it is important to draw a line and not get involved if you think there could be a conflict of interest.
Professor Amon: In my opinion, it is important to communicate clearly from the beginning and to maintain transparency to build mutual trust. You should explain why you chose a company for potential collaboration, but you should also mention if you cooperate with other companies. The other collaborations should not be in the same competitive field of the company you approach with an idea. Fortunately, it has become mandatory in scientific presentations or publications to disclose all cooperation and potential conflicts of interest.
Dr. Ang: People generally have more than one friend and working relationship. What you talk about and work on can be segregated into different compartments. It is important to maintain confidentiality.
Dr. Doane: I prefer to work closely with only a few device companies with the intention of completing clinical studies. My preference can be explained with an analogy. Some individuals may love attending big parties and saying hello to 100 people but not having a conversation. Others may love going to dinner with a small group of people and having an in-depth discussion. I prefer the latter scenario. It fits what I want to do with innovative concepts.
Dr. Donnenfeld: The key to working with multiple companies is to be transparent. Above all else, I make certain that what I am spending my time researching has the potential to be a best-in-class product. I also try to be fair and balanced to preserve my integrity as a clinician. I keep patients’ interests at the center of my mission, regardless of the outcome for the company partner. Following this mantra has served me well when working with industry.
Dr. Farid: A lot of companies are in the dry eye space nowadays, whereas there was really only one company maybe 15 years ago. With so many different products available and more in the pipeline, there is a lot of potential to grow the sector. I don’t think that companies are cannibalizing each other but rather raising awareness about the disease state and offering different solutions from different angles. This elevates our ability to treat patients. I don’t find consulting with different companies in the dry eye space problematic because I feel they all offer something slightly different. We’re collectively helping to move the field forward.
Dr. Gabrić: Verbal transparency is important. For example, our center was approached by a company to work on a product that was competing with the product we were currently working on. We contacted the CEO of the company with which we were already working and told him about the offer and explained that we wanted to continue working with his company. He understands that everyone wants to work with great doctors. I think that demonstrating transparency about your allegiances and work and honestly stating the pros and cons of every device you work with can increase your influence.
There are companies that want you to pretend that other companies do not exist. I think this is bad for clinicians and for the entire industry. Physicians must provide the best possible care to patients. A company that does not want doctors who work with them to speak about other manufacturers’ products can compromise patient care.
Dr. Klabe: I try to maintain a broad understanding of innovations in ophthalmology. My focus is always to contribute to serious and critical discussion of new solutions. In my opinion, this kind of balance is necessary when working with a specific company or multiple companies.
Dr. Kleinmann: I am amiable to working with multiple companies provided I am not working on the same sort of project or idea for them simultaneously. Regardless, it is important not to transfer data and information between the companies.
Dr. LaHood: Early in my career, I posed a similar question to Ronald Yeoh, MBBS, at a Future Opinion Leaders forum hosted by Carl Zeiss Meditec. When a presenter had a long list of financial disclosures, I imagined they would say that every new product was the best thing ever. That may be true of some clinicians, but Ron responded that working with multiple companies can actually make a physician less biased.
A pet peeve of mine is when the representatives of one company promote their product by denigrating a competing product. I therefore base my own commentary on my experience and avoid negative remarks about competing products or techniques unless I have supporting evidence. Companies know that I will talk about products I use and trust without thinking that only one company can make all of the greatest products.
Dr. Mansouri: At one point, I was the chief medical officer of two companies simultaneously, Sensimed, manufacturer of the Triggerfish continuous ocular monitoring system, and Implandata, manufacturer of the Eyemate implantable sensor for long-term IOP monitoring. Some people within the companies viewed each other as competitors. I had been approached by one when I was already the chief medical officer of the other. The expectation of the second company was that I would leave the first. I did not want to, however, because I believed in the first company and felt a sense of loyalty to it. I also believed in the new company.
I felt that my experience could help both companies, and I did not view them as competitors because, although they were in the same field, there were differences in their products’ target audiences and durations of action. Integrity and reputation are always important but even more so in a situation such as this. The contracts and nondisclosure agreements oblige you to respect confidentiality, but that is not enough. You must also be perceived as ethical and respectful of each company’s interests and expectations. I believe that, if these guidelines are followed, it is sometimes possible to work with competitors. The other big issue is time management. Can your schedule accommodate the assignments?
Dr. McCabe: I don’t see anything wrong with consulting for a lot of different companies. I consult with many companies that manufacture presbyopia-correcting IOLs, femtosecond lasers, and drugs, for instance, that compete with each other. The key is to be honest about your relationships with competitors and honor all confidentiality agreements. As with all relationships, transparency and honesty help me navigate the waters of industry collaboration.
Dr. Mehta: It’s important to be upfront with all the companies that you work with so that they know you work with other companies and in what areas. You can be honest without breaking confidences about the research and development of proprietary technologies. It is also important to be clear about what you are and are not comfortable doing.
Dr. Newsom: No one company does everything the best, so collaborating with multiple companies is exciting because it allows me to learn about a variety of new technologies. What works for one patient is not necessarily going to work for the next one, so I balance working with multiple companies by selecting projects that I think will move the needle for different groups of patients.
Dr. Okeke: I tell companies upfront that I am a physician and surgeon first and an educator second. I always want to choose what is best for my patients, and I am unwilling to form an alliance that requires I use only one company’s products. When choosing companies to work with, I am open about my consulting relationships and my prioritization of patient care and education.
Dr. Patel: Industry influence is the greatest concern. We clinicians want patients to trust our judgment, and we strive to remain unbiased while working with industry partners. I am cognizant that even the smallest gesture of goodwill can influence behavior, so I have instituted safeguards. Additionally, the time required to partner with a company can be significant. I like to be sure I can devote enough time and energy to each partner with the appropriate help achieve their goals. Partnership with industry provides many national and international travel opportunities, but these must fit into my busy clinical and family schedules.
Dr. Rosenberg: Being involved with industry requires time. I am fortunate to have a better half who also is an ophthalmologist, Alanna Nattis, DO, FAAO, and we work together to balance our professional endeavors and personal responsibilities such as caring for our 10-month-old child.
Working with multiple companies requires knowing your limitations. I don’t say yes to projects I cannot undertake, and I never assume that, because other people are doing something, it is easy. I allot the appropriate amount of time for the development of one thing. At the end of that project, I assess how it went, determine whether I can do more, and proceed accordingly.
Dr. Seibold: I think you must limit how much you work with any one company to avoid becoming biased. By working with multiple companies, you can stay more impartial and patient-centered in your daily care. It is also important to focus on how you can help each industry partner maximize the benefits that their products can provide to patients.
Dr. Smith: I prefer to have conversations with industry rather than exchange email messages back and forth because we get more done. Juggling can be hard. Sometimes at national meetings, marrying the academic commitment to industry interaction can make it really busy the entire time. My schedule is usually booked from the second I land until the second I leave. I have somebody help me with my calendar to make sure there are no conflicts, and I try to set aside a particular time in my schedule for industry meetings.
It is also important to balance your home life with the work that you do, and sometimes that requires you to find help from other sources so that all bases are covered.
Dr. Stonecipher: It took me a long time to figure it out, and I credit Richard L. Lindstrom, MD, for his guidance on the subject. He told me that he would consult on only one topic with a given company. It is crucial not to break nondisclosure agreements. If another company approaches me about the same topic, I am upfront about the conflict of interest. I say that I would be happy to answer questions about their product but cannot discuss other products in the field.
Dr. Waltz: The centers I work with are currently participating in 10 industry research projects in Central America. Most of the companies have no idea what the other projects are. We hide all of the documents of the other teams when a sponsor visits a facility. What industry cares about is if we respect them and give them the time that they need for things like enrollment and follow-up. Sometimes they find out years later that we were involved simultaneously in a project for another company. We also avoid participating in competitive trials to speed enrollment. Operating in secret can make it difficult to grow your reputation, but over time the caliber of our work and our discretion become recognized. This builds confidence and can attract new opportunities.
Company sponsors do not want you to talk about projects if they are a success or if they are a failure. If you get a reputation for blabbing, you won’t be asked to collaborate again in the future.
CRST: What benefits have you enjoyed through your collaboration with industry?
Dr. Ahmed: It is a joy to help patients and feel a part of bringing new technology and disruptive innovation to the field. I can help a patient with my own hands, which is great, but helping create something that could affect millions of people indirectly is highly gratifying. Early access to technology is another benefit. Collaborating with industry gives me a peek into the future, which is exciting and allows me to prepare for what’s to come. Having a say in how things are brought to the field is exciting.
Lastly, some of my closest friendships are with industry colleagues. My greatest motivation in doing things is the ability to bring people and ideas together.
Professor Amon: The main benefit has been expanding my knowledge. I have learned a lot about the production of implants and other devices, differences in biomaterials and their handling, details of sterilization, logistics, and regulatory guidelines.
A second benefit has been visiting different facilities in different countries and getting to know many interesting people working for industry in various capacities—from R&D to production to sales and marketing to management. Even my scientific research and creative drive have been stoked by the collaboration.
Dr. Ang: I enjoy sharing ideas. It is rare to have a say in which products make it to market. Additional benefits are speaking about new products and publishing articles on them. Being a part of the history of a product that benefits many patients is a good feeling.
Dr. Doane: My collaboration with industry on clinical research has enabled me to build some great relationships. Group experiences with incredibly bright nonphysicians and physicians have been stimulating. Moving the needle of innovation together has been enjoyable and inspiring. Collectively, we can make a difference and leave medicine better than it was when we began.
Working with industry has also added spice to my clinical practice, which at times can seem boring. Moreover, working on innovative products has given me the first look at a technology, sometimes 5 or more years earlier than I would otherwise. Lastly, using the technology in the relatively safe confines of an FDA study can give me a good sense of whether it is something I want to bring into my clinical practice.
Dr. Donnenfeld: For me, there’s nothing more exciting than developing a novel approach to a problem that’s going to change the way patients all over the world are taken care of. It’s satisfying to be involved in promoting industry advances that circle the Earth and are used on thousands, if not millions, of patients. This is the most rewarding part of working with industry. When I take care of a patient in my office, I can make a difference in their life. When I work with industry, I can make a difference in literally hundreds of thousands of patients’ lives. I also derive a significant sense of accomplishment from being an early adopter of successful technology. It can establish you as an expert in the field.
Dr. Farid: I have met so many people from the science, marketing, and business arms of the ophthalmic space and have learned from so many amazing minds. Industry colleagues may move from one company to another, but many times, they stay in ophthalmology. Lifelong friendships are one of the best parts of working with industry.
Dr. Gabrić: The first benefit is financial—you can get a free device, free procedures, compensation, and paid travel arrangements. It’s tempting to think that’s the best part and easy to quantify, but what moves me is the knowledge that something I do will help my colleagues and thousands or, in some cases, millions of patients. You can’t buy the feeling of knowing that you are part of the DNA of the product and a part of you is in every procedure that’s performed. Maybe it was a little button, maybe it’s a new method of docking, or maybe it’s suggesting a new angle for the spatula. Something you’ve contributed to the innovation will change lives and vision for patients around the world.
Dr. Klabe: The modern practice of ophthalmology would not be possible without the latest technological and pharmacologic approaches. Many of them are a far cry from what I learned in medical school, residency, and fellowship. Collaborating with industry keeps me updated on the latest advances in care and allows me to take part in innovation.
Dr. Kleinmann: I have learned to think outside the box. Many ideas in ophthalmology come from other disciplines. It gives me joy to connect people from different fields with the idea that their collaboration could drive innovation in either or both fields. Another benefit is being involved in developing something that I expect to become available and advance the field in 5 to 10 years or even more.
Dr. LaHood: I love discussing research ideas with like-minded individuals at the top of their game. Industry attracts the best and the brightest from the fields of optics, orthoptics, optometry, and ophthalmology. Talking about hypotheses, pushing the boundaries of what can be achieved, and learning from others is an incredible opportunity.
Additional benefits are travelling to places I would not visit otherwise, previewing innovations, and sometimes gaining early access to products that can benefit my patients.
Dr. Mansouri: One benefit is gaining access to technologies before they are commercialized, such as during clinical trials or a soft launch. This allows me to give my patients access to technology before others can. Working closely with a company entails getting to know its people. Sometimes they are willing, when I ask, to provide the technology for free or at a reduced price to patients who need but cannot afford it or for a mission in a developing country.
Another advantage is that I learn by doing research. I improve my clinical abilities, and the collaborations can make my day-to-day work even more interesting. Participating in these research projects can also draw younger colleagues to my center to train with me. I can help select colleagues, including former fellows, for clinical trials.
A more personal benefit is when colleagues view me as an innovator and become more likely to refer patients to me because they believe their patients could benefit from technologies that I can offer early on or from my insight into technologies that may be available only outside the country.
The disadvantages are less free time and more headaches because not only am I managing my clinic and employees, but I am also assisting with the management of people in different settings.
Dr. McCabe: Collaborating with industry has enriched my experience as a physician more than most things because I’ve had insight into and helped shape new developments and advanced our field in ways that can benefit patients. Some of my best friends are people from the industry side whom I’ve gotten to know well and spent a lot of time with on projects that we mutually care about. This adds a new dimension to the impact I can have on ophthalmology and future patients.
Dr. Mehta: Collaborating with industry has allowed me to deliver lectures and participate in teaching academies all around the world and meet a lot of like-minded people in corneal and refractive surgery. I have also had the pleasure of being a leader in many innovative products that have come to market.
Dr. Newsom: One benefit is the knowledge I gain about a technology and what it is doing. I get to learn about the current and possible problems. In the process, I learn how to maximize the potential of technologies and how to push past their limitations.
It is neat to see some projects that I worked on 10 years ago become the latest disruptive technology. For example, in the past, IOL technologies were fixed. No one thought a lens could be changed after implantation in the eye. Then, the Light Adjustable Lens (LAL; RxSight) was developed. Current questions are how to make this IOL work even better. A similarly disruptive technology is the SBL-3 IOL (Lenstec). My colleagues and I implanted the first of these segmented bifocal lenses in the United States a month ago. In a year, we will know much more about how best to use the technology.
A third benefit is an opportunity to mentor the next generation of surgeons. I involve my fellows in my industry research projects, which allows them to make connections. My hope is that they can form their first great partnership 1 or 2 years into practice versus the 10 to 12 years it took me.
Dr. Okeke: One benefit is being at the forefront of technological advances. Another is feeling that I am contributing positively to the care of large populations—more patients than I could ever see by myself. A third is networking opportunities, which I find fulfilling (Figure 4).

Figure 4. Dr. Okeke providing wet lab instruction to an attendee at MillennialEye Live, the annual meeting of the unique all-digital publication and online community, and Bryn Mawr Communications. In 2023, the meeting is becoming YoungMD Connect Live, powered by the YoungMD Connect community.
My creative ideas, including my iGlaucoma YouTube channel (www.youtube.com/c/iGlaucoma; Figure 5) have also benefitted from industry support. All in all, my work with industry allows me to enjoy a lot of variety in my career.

Figure 5. The iGlaucoma YouTube channel hosts video series like MIGS Success Surgical Secrets.
Figures 4 and 5 courtesy of Constance Okeke, MD, MSCE
Dr. Patel: I enjoy seeing an idea develop into a treatment or product. As an end-user physician, I was unaware of all the development and business work required to bring a product to market until I partnered with industry. Navigating the innovation process, federal regulations, and reimbursement issues helped me understand why industry partnership is valuable.
Collegial interaction has been one of the best unexpected benefits. The exciting clinical conversations have opened new pathways of support for some of my most challenging cases. Some of my collaborations have led to opportunities to develop and evaluate glaucoma technologies. Being an early adopter of certain technologies has also been valuable to my clinical practice and reputation.
Dr. Rosenberg: I enjoy bringing new products to my patients. I also like learning about, implementing, and teaching residents about new technologies. Groundbreaking technologies are not developed and disseminated by people who want to stick with the status quo. I want to show young surgeons why it is important to get involved in the early stages of product development.
Dr. Seibold: One benefit has been my interactions with other physicians. They are often some of the greatest surgeons in our field, and I learn so much from them. Some of my most treasured friendships with colleagues were formed through my collaborations with industry. Another benefit is early access to novel innovations through device testing and preclinical studies.
Dr. Smith: I love working with industry. Some people say that you work with industry to get paid, but for me, that is not it. The first benefit I would identify is the ability to help improve and enhance the quality of care we are able provide to patients through innovation for my profession. The second is exposure and opportunities to participate in clinical research. This gives me some expertise by the time a product hits the market.
A third benefit is relationships. Because I know members of industry personally and have made myself available for discussions, it is easy to reach out to them when I am seeking a sponsor for an event or a partner on a project. For instance, New World Medical partnered with the Care Glaucoma Foundation on a pilot study a couple years ago.
Lastly, I have greater access to information. When I need slides or study information for a presentation, for example, I can contact someone at a company I have worked with for assistance.
Dr. Stonecipher: People in industry move around; they change positions and companies. A lot of times, I leave in my phone’s contact list where people started so that I remember how I first got to know them. The biggest benefit of the collaboration is you get to meet new people and make new friends, and then they stay with you in industry and ask you to do other cool industry-related things.
Dr. Waltz: One first benefit is getting to know interesting people. People in industry have different goals and skills than doctors. Interacting with them broadens my perspective on the world. Some of my favorite people are in industry. I respect what they have done for the field of ophthalmology and my individual career.
At a high level, industry collaboration is about friendship and trust. During our projects, we essentially climbed mountains together. Sometimes we reached the summit. Sometimes we did not, but we had an adventure and found rewards along the way. The shared challenges become a strong bonding experience.
CRST: What are some of the most memorable collaborations you’ve had and why?
Dr. Ahmed: Changing the field of glaucoma dramatically and disruptively—and we are still in the middle of it—into an interventional specialty with MIGS and other approaches has been a collaboration between clinicians and surgeons, industry, engineers, and banks and investors. All have their own motivations, but we continue to come together to change the field (Figure 6). I do not want to be negative, but glaucoma was traditional for decades and surgical innovation was sparse. Now it is a large field that is changing and drawing a lot of interest, including from medical students and residents and from entities investing millions of dollars.

Figure 6. Dr. Ahmed with fellow Interventional Glaucoma Consortium (IGC) program chairs Arsham Sheybani, MD, and Richard Lewis, MD, at the 2021 annual meeting. The IGC is a meeting hosted by Bryn Mawr Communications and Glaucoma Today that uses a think tank–style format to encourage leading glaucoma specialists to exchange innovative ideas and education that promotes a proactive approach to patient care.
Courtesy of Pamela Marans Katz
Professor Amon: My long-lasting collaboration with an IOL manufacturer is the most memorable. Getting the chance to invent an IOL for worldwide release, create an approach to presbyopia correction, describe the Duet procedure with the Sulcoflex (Rayner; Figure 7), be the first surgeon to use the implant, and present the results on a scientific level was deeply satisfying.
Figure 7. The Sulcoflex multifocal toric (A) and trifocal (B) IOLs.
Another wonderful experience was collaborating with Geuder on the development of the Forceps-Needle (Figure 8), an instrument that facilitates scleral fixation of IOLs.
Figure 8. The Forceps-Needle, which Professor Amon developed with Geuder.
Figures 7 and 8 courtesy of Michael Amon, FEBO
Dr. Ang: Long-term collaborations are memorable because I have been there to help the innovation evolve from the beginning. Eight years ago, I was the second surgeon to implant an IC-8 small-aperture IOL (now IC-8 Apthera, AcuFocus) in a patient. I have worked with the engineers and technical team at Bausch + Lomb for 20 years, including on the Victus, Technolas Teneo, Supracor, and transepithelial PRK. The optical scientists at PhysIOL taught me a lot about diffractive optics and aberrations, and I use the knowledge I gained when evaluating IOLs.
Most memorable are the people I have met and worked with who have become lifelong friends.
Dr. Doane: I was involved collaboratively in the first in-human LASIK procedures with advanced lasers and software at the beginning of my career in 1995/96 outside the United States. It was incredibly stimulating to see something work for the first time. In the process of medical in-services, I was able to visit and treat patients on three continents that I had not yet visited. It was an eye-opening experience personally and intellectually. The advances were breathtaking, and the innovators were sharp (Figure 9).

Figure 9. Being intimately involved with clinical studies afforded Dr. Doane the opportunity to meet luminaries in the field, including Professor José Ignacio Barraquer.
My work on the Crystalens (Eyeonics, later acquired by Bausch + Lomb) is memorable because of the technology itself, the physician skills transfer, and seeing a company that had one product move forward with urgency, unmatched energy, and intention (Figure 10).

Figure 10. Dr. Doane with Stephen G. Slade, MD, FACS; Steven J. Dell, MD; and Andy Corley. The three doctors are intimately involved with the American-European Congress of Ophthalmic Surgery (AECOS), whose mission is to coordinate relationships between surgeons, inventors, and industry to advance technological innovation for the betterment of patients’ vision and outcomes. An important offshoot of the doctors’ combined work with Eyeonics was Mr. Corley’s leadership to pass CMS Presbyopic IOL Policy Ruling No. 05-01, issued May 3, 2005. Without this ruling, the incentives for innovators to create new IOL technologies would be lacking.
Figures 9 and 10 courtesy of John F. Doane, MD, FACS
Another standout experience for me was my involvement in introducing a new surgical procedure, SMILE, with the VisuMax femtosecond laser (Carl Zeiss Meditec). A decade of study was required to overcome the technological and optical hurdles. During this time, I watched other teams not be able to duplicate what the team I worked with was able to achieve.
My collaboration on the LAL shares attributes of the experiences I just described with one important difference: All the others had predicate uses. LASIK had excimer lasers and PRK, the Crystalens had prior IOLs, and SMILE had femtosecond LASIK flaps. The LAL required the advancement of novel photochemistry to address the unpredictability of IOL effectiveness. The lens material was designed by the late Robert H. Grubbs, PhD, who was a corecipient of the Nobel Prize in Chemistry in 2005 for his work on olefin metathesis. In hindsight, what Daniel Schwartz, MD, invented with this technology seems almost inevitable, but in addition to his inventiveness, it required the innovation of Dr. Grubbs to transfer this technology to ophthalmology and clinical practice, which was a massive move forward for eye surgery.1
Dr. Donnenfeld: There are too many memorable collaborations for me to list. I was an initial investigator for the Visx excimer laser, the femtosecond laser for cataract surgery, CXL, numerous pharmaceuticals, immunomodulators for dry eye disease, the first MIGS device, and multiple IOLs. All these technologies started out as ideas shared between clinicians and industry, and they have changed the face of ophthalmology.
When I look back at the way we performed cataract and refractive surgery when I was in residency and compare it to what we’re doing today, the advances I have witnessed are almost inconceivable. All of them came from the relationship between ophthalmologists and industry. I’m proud that I was a part of various innovative collaborations that brought disruptive technologies to the market.
Dr. Farid: I am a member of Ophthalmic World Leaders (OWL), which promotes diversity in leadership. The most recent OWL Catalyst Award was presented at the 2022 AAO meeting to Magda Michna, PhD, the chief global clinical, medical, and regulatory affairs officer at AcuFocus. She has a brilliant mind. She is also a real innovator and an amazing person to work with and learn from. The relationship we built around the work that she has done is precious to me. That relationship and others like it are what is most memorable about collaborating with industry.
Dr. Gabrić: Most memorable is usually the one that is most recent, but it’s been the Atos project with Schwind eye-tech-solutions. During the pandemic, only three of the devices existed—one in Nepal that required service, one in Switzerland that the researcher was not using during the lockdown, and one at our center. We were the first to perform procedures with the Atos in Europe and had nobody to call if something went wrong because nobody had the experience to help us. Doing something first and knowing there was no one and nothing to support us but our own imagination was amazing.
IOL and cataract procedures are standardized—just variations on a theme. Working on devices for corneal refractive surgery is different. You are inventing the rule book. The most exciting part is later telling colleagues about the machine and how to solve a problem.
Somebody has to be first. We can be followers, or we can be trailblazers. Many surgeons become more conservative as they age. Young surgeons and those who remain on the cutting edge have an opportunity to change the field.
Dr. Klabe: Rather than one special moment, it is my continuing work with Oertli. More than a decade ago, I was asked to join the medical team in developing surgical platforms. Since then, I have been involved in the development of a complete family of surgical platforms for anterior and posterior segment surgery. My long and close collaboration with other physicians, engineers, technicians, mechanics, and businesspeople remains unique.
Dr. Kleinmann: After a project concludes, I enjoy reflecting on the late hours my collaborators and I spent and the progression from a drawing on a piece of paper to studies to a product on the market. An example is the long project to develop the CleaRing (Hanita Lenses), a device to prevent posterior capsular opacification. Attending an ophthalmology meeting sparked an idea in me that I brought to the company. I worked with Hanita’s engineers to shape the idea, test the resultant product in animal models, and implant it in humans.
Dr. LaHood: My work with Alcon gave me an opportunity to host an amazing podcast series, “The Second Look,” and chat with renowned ophthalmologists. Not only was it fun, but the podcast also allowed me to get to know the people behind the headlines. As a result, I was able to ask Carol L. Shields, MD, for help with a patient who had melanoma and participate in teaching sessions with Ivo Ferreira Rios, MD. I am grateful that my relationship with Alcon led to my running their podcast, and more episodes are coming soon.
Another memorable collaboration was travelling to Xi’an, China, to help launch the AT LISA tri IOL (Carl Zeiss Meditec). I had long been fascinated by the terra-cotta warriors of Emperor Qin Shi Huang, so seeing them was a dream come true (Figure 11). I will never forget the experience, the food, and all the people I met on the trip.

Figure 11. Dr. LaHood in Xi’an, China.
Courtesy of Ben LaHood, MD, MBChB(dist), PGDipOphth(dist), PhD, FRANZCO
Dr. Mansouri: The most memorable—in part because it was the longest and I was young when the collaboration started—was with Sensimed. The Triggerfish was innovative. We could really see what happened with patients’ IOP while they were at home and during their daily activities. We learned a lot about patients, including things we did not expect, and the epigenesis of glaucoma. It led to my delivering the first TEDx Talk in ophthalmology (Figure 12; scan the QR code to watch Dr. Mansouri’s talk).
Figure 12. Dr. Mansouri delivering his TEDx Talk, “Your Eyes Are the Gateway to Your Soul - Affect/Possibility.”
Dr. McCabe: I enjoy trying to help companies realize their ideas. Specifically, I like to work with small companies with novel ideas as well as larger companies that I believe to be on the cutting edge. In particular, I am passionate about presbyopia-correcting IOLs and have participated in several early research studies for Alcon (see What Does It Take to Be Involved in a Clinical Trial?). I also enjoy contributing to the thought process for communicating the benefits of new technology to my colleagues. Through my experiences with both basic science and marketing teams, I have helped bridge the knowledge gap—science to simplification, if you will.
What Does It Take to Be Involved in a Clinical Trial?
By Cathleen M. McCabe, MD
A surgeon’s first involvement with clinical trials can be an eye-opener. Much more goes into participating than simply collecting data. Luckily, most companies have programs that educate individuals on the nuances of clinical trial work before they get started. Below are four pointers I learned from attending one of these programs.
- No. 1: Level-set your team. Involve your team in the learning process. When a company invites me to a clinical trial seminar, my entire team goes with me. Some companies will also travel to a practice to help train new members of the research team.
- No. 2: Appoint a research team. This includes a research coordinator, who interacts with the trial sponsor and keeps track of the data and other trial requirements, and a staff member who is responsible for the collection of preoperative data. Sometimes, the research team consists of people already within your organization; other times, new team members must be added.
- No. 3: Negotiate a budget. Calculate the overhead costs of running a clinical trial in your practice and negotiate with the clinical trial sponsor to cover the budget.
- No. 4: Learn how to identify and enroll patients. Communicating with patients that they are good candidates for clinical trial enrollment takes practice. You must learn how to explain the trial to them in a way that is comprehensive and transparent and entices them to enroll.
Dr. Mehta: Most memorable are ones that allowed me to demonstrate innovation that has changed the field (Figure 13). For example, I traveled to Dubai 12 years ago to give a talk on what at the time was Carl Zeiss Meditec’s new lenticule extraction procedure. As I left the podium, attendees asked me, “Does it really work?” I was able to respond, “Yes, these results are real. They were from my own patients.” Now, almost 6 million refractive lenticule procedures have been performed.
Figure 13. Dr. Mehta performs live surgery in Singapore during Ziemer Academies, an event hosted by the company.
Courtesy of Ziemer
Another memorable experience is the work I’ve done with femtosecond laser–assisted pterygium surgery. In the beginning, the procedure was just a concept that we did some wet lab and animal studies on. What is truly memorable for me is that Ziemer had not thought it possible, but they were willing to work with us to develop it. The results have been outstanding, both with recurrence rates and cosmesis.
Dr. Newsom: The most memorable collaborations are those that bring value to patients. They produce technologies that fix a problem. For me, these include the AcrySof IQ PanOptix and Vivity lenses (both from Alcon), the LAL, and potentially the SBL-3 IOL. Each moves technology to the next level.
Dr. Okeke: Some of the most memorable were the earliest collaborations. My first real consulting role was with NeoMedix, which expanded to so many different opportunities. My early clinical trial work with the iStent (Glaukos) helped me to become more innovative. Another memorable experience was traveling to the Alcon Experience Center for the company’s strategic advisory council meeting. It was the first time I was ever invited to something like that, and I was honored. I was still early in my career and contemplated not going because I did not want to miss a surgery day. In the end, I decided to attend, and I felt proud of myself that I came into the space and contributed to the conversation. I remember one of my peers saying, “You deserve to be here. You are a major contributor to this meeting.” These words still stick with me today because it is a great reminder to never undervalue your contributions.
Dr. Patel: My initial collaborations brought quantifiable but superficial returns, whereas the later ones have deepened and broadened my professional life.
Partnership with Nova Eye Medical has been a lasting experience. Initially, it helped me refine my surgical technique and postoperative outcomes with the iTrack and Molteno shunt. Over the years, I also became involved in the development of protocols for the company’s national and international studies. One of my greatest memories is spending time with fellow surgeons and members of the company in Panama, where we all helped refine the surgical technique for the iTrack Advance, a new handpiece for internal canaloplasty (Figure 14). Outside the OR, the casual clinical conversations around the dinner table or during shuttle rides were riveting and memorable.
Figure 14. Dr. Patel operates on a patient in Panama.
Understanding the sophisticated and logical thought processes of colleagues provides an opportunity for growth. Industry partnership is another channel through which I can lean into growth, all for the benefit of patients.
Dr. Rosenberg: My first collaboration was on the intracameral delivery of phenylephrine 1.0% and ketorolac 0.3%. I participated in a retrospective case review with Dr. Donnenfeld and others.2 As we read hundreds of charts, we began to appreciate findings in new ways. For example, a secondary outcome measure of the study was that complication rates increased when visualization was poor. It reaffirmed something we all knew but presented it in a different way.
Another memorable experience has been working with digital visualization and cofounding the Digital Ophthalmic Society (DOS). The DOS is a thought engine centered around novel digital solutions with clinical applications, including digital surgical visualization, digital health solutions, image-guidance systems, telemedicine platforms, and other emerging technologies. Working together with other digital technology experts, the DOS hopes to help colleagues hone their skill sets and provide tools that help them stay at the forefront of digital evolution.
Dr. Seibold: My most memorable collaborations have been in the MIGS space. These procedures are the most exciting and meaningful part of what I do for patients, so having early access to the procedures and helping to guide revisions and future directions in the space have been meaningful.
Dr. Smith: One of my most memorable collaborations is working with Allergan. During our partnership, I have seen glaucoma treatment evolve from primarily medication to a mix of pharmaceutical and surgical interventions. It is satisfying to know that I am helping to advance patient care.
I also get great satisfaction from the aforementioned pilot study with New World Medical. The research and humanitarian outreach are making a difference in the education of glaucoma surgeons worldwide, and through these efforts the company is supplying drainage implants for patients in need.
I’ve also been fortunate to travel to other countries to try out new surgical devices (Figure 15). The marriage of gaining a new skill set, learning from peers, and seeing a new place is rewarding. I always come home excited about the possibilities for the technology.
Figure 15. Dr. Smith operates on a patient in Panama.
When industry listens to us physicians and makes the changes that need to be made for better patient care—whether that is changing how an applicator works or the concentration of a drug—knowing you were part of the improvement gives you satisfaction.
Dr. Stonecipher: My favorite kind of collaboration is one where everyone can let their guard down, where I am really among friends. We may disagree on things, but no one takes it personally, even though ophthalmologists can get competitive.
One of my most memorable collaborations is the annual wine party I host at the Hawaiian Eye meeting. That started out as a collaboration with friends, including David Hardten, MD, and Helen Wu, MD, and grew into a bigger event that I now cohost with Bryn Mawr Communications, parent company of CRST.
Dr. Waltz: Almost 5 years ago, I received a call from individuals at Clerio Vision who, based on a referral, wanted to collaborate on research with me but were nervous about doing it in El Salvador. I encouraged them to meet me at Clínica Quesada, my organization’s partner in San Salvador, so that I could give them a tour and demonstrate our capabilities.
In January 2018, Daniel Summers, then Clerio’s director of development, operations, and compliance, met me in San Salvador. After touring the facility, he excused himself to make a call. While waiting for him to return, I received a call from Nick Tarantino, OD, FAAO. He and Dan had worked together at Abbott Medical Optics. Nick told me he had just spoken to Dan and assured him that partnering with my group was the right choice. Nick also reassured me Dan would be a good partner. We hung up, and Dan returned to tell me that he had spoken to Nick and trusted his judgment.
The project got underway. My colleagues and I agreed to create a study protocol for a laser that Clerio had not yet created in a special OR that we had not yet built. By October 2018, the protocol had been approved, the OR had been constructed, and the laser had been built and imported—all on a handshake. It was one of the coolest projects I ever worked on (Figure 16).
Figure 16. A Clerio Vision corneal refractive treatment viewed under special lighting to enhance the barely visible changes. The procedure was performed October 2018 at Clínica Quesada, San Salvador (A). Dr. Waltz and the Clerio Vision team after surgery at Clínica Quesada, San Salvador (B).
Courtesy of Kevin L. Waltz, OD, MD
Another memorable experience was a collaboration with Aurion Biotech in 2020. The goal was to implant cultured corneal endothelial cells in a large group of patients to treat corneal endothelial disease in lieu of performing corneal transplantation or endothelial keratoplasty. What the company wanted us to do in San Salvador was difficult. Live cells had to be cultured and grown in Japan, then transported from Japan through the United States to El Salvador during the COVID-19 pandemic. Permits were acquired, the packaging was temperature-controlled to maintain cellular viability, and the shipment was tracked with GPS. Hurricane Iota hit San Salvador on the day we were supposed to operate. We knew the cells would die if there was a delay. Six US surgeons and eight representatives of the company traveled to San Salvador on time. The cells were implanted, and the patients did well. It was a remarkable combination of talents to achieve a common goal.
1. Schwartz DM. Light-adjustable lens. Trans Am Ophthalmol Soc. 2003;101:417-436.
2. Rosenberg ED, Nattis AS, Alevi D, et al. Visual outcomes, efficacy, and surgical complications associated with intracameral phenylephrine 1.0%/ketorolac 0.3% administered during cataract surgery. Clin Ophthalmol. 2017;12:21-28.