Prostaglandins, produced by the COX-1 and COX-2 receptor pathways, are the enemy of cataract surgery. They induce inflammation; break down the cell membranes; and can cause postoperative cystoid macular edema, pain, and photophobia.1 The AAO Preferred Practice Pattern Guidelines for Cataract in the Adult Eye and the ASCRS Refractive Cataract Surgery Subcommittee guideline both suggest that effective preoperative mydriasis is important to help minimize risks that accompany a small pupil and intraoperative miosis.2,3 Mydriasis is typically achieved with topical and/or intracameral administration of anticholinergic agents, sympathomimetic agents, or both. Other common treatments including pre- and postoperative topical NSAIDs or steroids can also be prescribed to reduce inflammation, surgical pain, and the risk of cystoid macular edema. Although preoperative and/or intracameral topical NSAIDs can help maintain intraoperative pupil tone, the therapeutic effect may be reduced or eliminated during surgery if the irrigating solution washes the drug away from ocular tissues. OMIDRIA® (phenylephrine and ketorolac intraocular solution 1.0%/0.3%, Rayner) is the only FDA-approved product for delivering an NSAID (ketorolac) with the irrigating solution intraoperatively to inhibit inflammation, prevent intraoperative miosis, and reduce postoperative pain.4
We know that the most effective way to prevent the side effects of inflammation is to stop it from occurring in the first place. The use of OMIDRIA, the first and only FDA-approved nonopioid NSAID-containing treatment approved for use in cataract surgery, inhibits prostaglandin synthesis secondary to ocular surgery and prevents inflammation, resulting in quieter eyes with less surgically induced miosis. More importantly, patients see better and more quickly after surgery, and they have less risk of complications.5
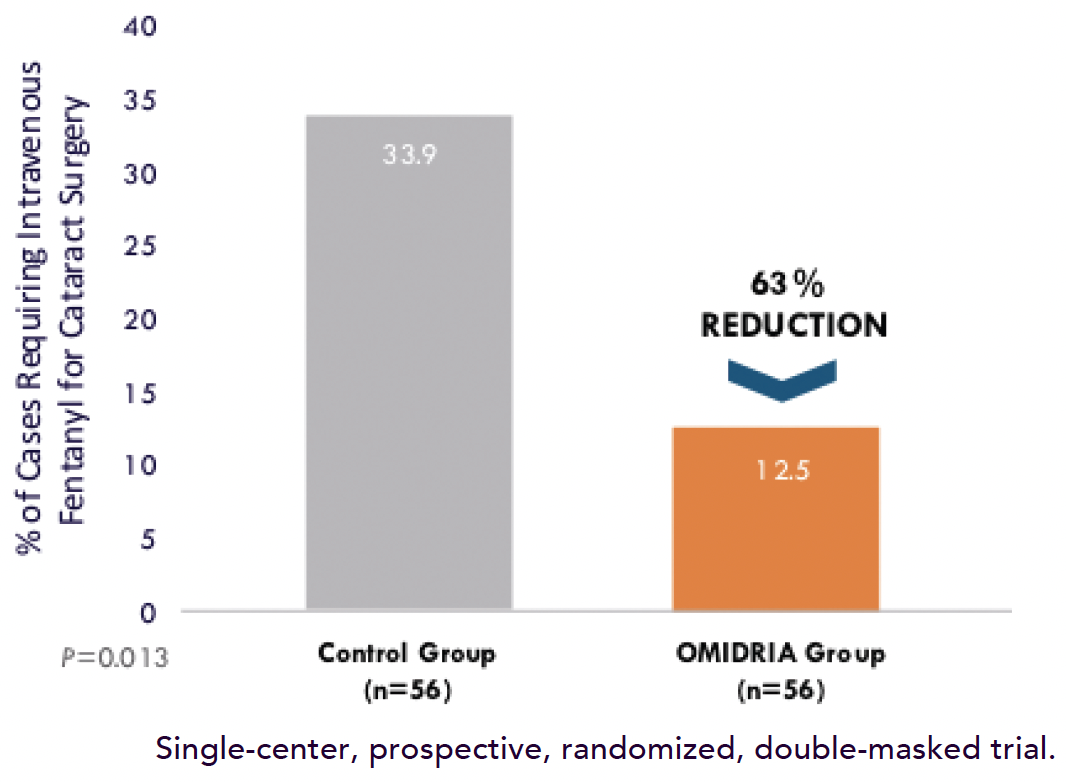
Figure. OMIDRIA was associated with a threefold reduction in intraoperative IV fentanyl use.
MAJOR BENEFITS
Studies show that the addition of an intracameral NSAID is one of the most important measures you can take to improve outcomes with cataract surgery.4-7 In my opinion, the three major benefits associated with the use of OMIDRIA are the following.
Major Benefit No. 1: Maintaining pupil size. OMIDRIA has superiority over the use of either ketorolac or phenylephrine alone in miosis prevention.8 It can maintain a larger pupil size intraoperatively. This leads to a higher patient comfort level, a faster surgical procedure, and a reduced risk of complications.4-7 In the Phase 2b clinical trial, only 6% of patients who received OMIDRIA experienced a reduced pupil size of less than 6 mm compared to 22% of patients who received phenylephrine alone. Phenylephrine is very similar to intracameral epinephrine, which is commonly used off-label by many ophthalmologists (see The Supply Chain).8
Major Benefit No. 2: A reduction in pain during and after cataract surgery. The FDA clinical trials also showed a statistically significant reduction in pain during and after cataract surgery associated with the use of OMIDRIA compared to phenylephrine.8-10 Additionally, patients were less likely to experience foreign body sensation. As a result, patients move less during surgery, and there is lower incidence of pupillary spasm. These benefits translate to better surgical results and a happier, more relaxed surgeon intraoperatively. Additionally, patients are more likely to share with their family and friends that cataract surgery is a comfortable, painless procedure. Patients can experience up to a 50% reduction in pain intraoperatively and are up to 50% more likely to be pain-free for up to 12 hours after surgery.11,12
Major Benefit No. 3: Less chance for an opioid to be used intraoperatively When patients experience pain during cataract surgery, it is not uncommon for anesthesiologists to use fentanyl intravenously. My colleagues and I have showed, however, that the use of OMIDRIA statistically significantly reduced the need for fentanyl or opioids to be used during the procedure (Figure).9,13
As cataract surgeons, we don’t think of ourselves as significant contributors to the opioid epidemic, but we’re operating on elderly patients—many of whom have been previously exposed to opioids. By maintaining a comfortable environment during surgery with the use of the intracameral NSAID ketorolac, we can play a significant role in helping to fight the opioid epidemic.9,13 In fact, the FDA has granted OMIDRIA a prolonged transitional pass-through payment status for this reason.
ANCILLARY BENEFITS
In addition to the major benefits, several ancillary benefits are also associated with the use of OMIDRIA during cataract surgery.
Ancillary Benefit No. 1: A reduced need for pupillary expansion devices. With the simple addition of intracameral OMIDRIA, the associated risks of iris damage and increased inflammation caused by surgical manipulation are eliminated.4,14-16
Ancillary Benefit No. 2: Patients require fewer topical medications postoperatively. About 20% of patients who have cataract surgery cannot instill drops themselves.17 For patients who receive a typical postoperative regimen including prednisolone acetate, an antibiotic, and an NSAID 4 times a day, they’re taking about 200 drops over a period of 4 weeks. Combining OMIDRIA with either Dexycu® (dexamethasone intraocular suspension 9%, EyePoint Pharmaceuticals) or Dextenza® (dexamethasone ophthalmic insert 0.4 mg, Ocular Therapeutix) can help reduce the drop burden, allowing patients to have a comfortable postoperative period without having to worry about medication compliance.
In a study presented at the 2022 ASCRS meeting and currently in press in the Journal of Cataract and Refractive Surgery, my colleagues and I showed that combining OMIDRIA with dexamethasone ophthalmic insert 0.4 mg and intracameral moxifloxacin eliminated the need for postoperative drops. Not only were patients’ visual results and macular thickening identical to the visual results in patients who were prescribed prednisolone acetate 1%, moxifloxacin 0.5%, and ketorolac 0.5%, but more importantly, 96% of patients preferred OMIDRIA to a routine drop regimen.
With the combination of OMIDRIA and dexamethasone ophthalmic insert 0.4 mg or dexamethasone intraocular suspension 9%, I know that patients are getting the required medications that they need. Another advantage is that all three of these products currently have pass-through payment status and essentially cost the patient nothing compared to up to $400 for routine drop regimens.
I think the time has come that we need to start thinking about new ways of providing better drug delivery to our patients. OMIDRIA, in my opinion, is one medication that is at the forefront of the charge to improve patient compliance and surgical outcomes.
Ancillary Benefit No. 3: OMIDRIA improves the patient experience. My goal as a refractive cataract surgeon is to maximize the benefits I can give patients while maintaining a safe surgical procedure. I think OMIDRIA plays a significant role in both aspects. I tell surgeons who don’t use OMIDRIA or feel it’s only necessary in difficult cases that it is the easiest way to prevent pupillary miosis in every case. As a result, the patient’s eyes are quieter, their vision is better on postoperative day 1, and they get the wow effect that every patient desires. I think there is a lot to be said for elevating the patient experience with a comfortable surgical procedure and extraordinarily good vision the day after surgery.
Ancillary Benefit No. 4: Ketorolac might be passed through to the vitreous. I strongly believe that the combination of OMIDRIA and modern cataract surgery leads, for the first time, to high levels of ketorolac in the vitreous rather than just in the anterior chamber. High-pressure forced infusion used for cataract surgery forces the irrigating solution with ketorolac through the zonules and into the vitreous. This can prolong contact time to the eye and act as a depot. It can also decrease the need for postoperative NSAIDs. I believe this is the reason why there is a reduced risk for cystoid macular edema when OMIDRIA is used compared to conventional cataract surgery drop regimens.10
THE SUPPLY CHAIN
In some areas, there is a shortage of epinephrine due to supply chain issues surrounding the COVID-19 pandemic. Rather than using another drug in an off-label fashion to control pupil miosis, which puts the patient and the doctor at risk, consider the use of OMIDRIA. This eliminates any unnecessary risk associated with the use of off-label drugs and increases compliance in your practice.
CONCLUSION
In my experience, adding OMIDRIA to all cataract surgery procedures allows my patients to experience a more comfortable procedure and makes an enormous difference in cataract surgery outcomes by reducing pupillary miosis, pain and inflammation, as well as the use of opioids during cataract surgery. Secondarily, the use of OMIDRIA reduces the need for pupillary expansion devices and can decrease the medication burden for patients.
OMIDRIA helps patients to see better, to feel better, and to ensure they undergo safer cataract surgery. This makes a big difference to surgeons because we can achieve more successful surgery with fewer complications, and it makes a difference to our patients, who are happier after surgery.
US-OM-2200035 11/22
1. Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of acute postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2007;17:20-28.
2. Miller KM, Oetting TA, Tweeten JP, et al; Academy of Ophthalmology Preferred Practice Pattern Cataract/Anterior Segment Panel. Cataract in the adult eye preferred practice pattern. Ophthalmology. 2022;129(1):1-126.
3. Al-Hashimi S, Donaldson K, Davidson R, et al; ASCRS Refractive Cataract Surgery Subcommittee. Medical and surgical management of the small pupil during cataract surgery. J Cataract Refract Surg. 2018;44(8):1032-1041.
4. OMIDRIA [package insert]. Seattle, WA: Omeros Corporation; 2017.
5. Rosenberg ED, Nattis AS, Alevi D, et al. Visual outcomes, efficacy, and surgical complications associated with intracameral phenylephrine 1.0%/ketorolac 0.3% administered during cataract surgery. Clin Ophthalmol. 2018;12:21-28.
6. Data on file with Rayner.
7. Lindstrom RL, Loden JC, Walters TR, et al. Intracameral phenylephrine and ketorolac injection (OMS302) for maintenance of intraoperative pupil diameter and reduction of postoperative pain in intraocular lens replacement with phacoemulsification. Clin Ophthalmol. 2014;8:1735-1744.
8. Donnenfeld ED, Whitaker JS, Jackson MA, Wittpenn J. Intracameral ketorolac and phenylephrine effect on intraoperative pupil diameter and postoperative pain in cataract surgery. J Cataract Refract Surg. 2017;43:597-605.
9. Donnenfeld ED, Mychajlyszn D, Mychajlyszyn A, Stein R. Pain control and reduction of opioid use associated with intracameral phenylephrine/ketorolac 1.0%/0.3% administered during cataract surgery. J Cataract Refract Surg. 2022;48(7):759-764.
10. Visco DM, Bedi R. Effect of intracameral phenylephrine 1.0%–ketorolac 0.3% on postoperative cystoid macular edema, iritis, pain, and photophobia after cataract surgery. J Cataract Refract Surg. 2020;46(6):867-872.
11. Donnenfeld ED, Shojaei RD. Effect of intracameral phenylephrine and ketorolac 1.0%/0.3% on intraoperative pain and opioid use during cataract surgery. Clin Ophthalmol. 2019;13:2143-2150.
12. Hovanesian JA, et al. Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain: Integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg. 2015;41:2060-2068.
13. Davidson RS, Donaldson K, Jeffries M, et al. Persistent opioid use in cataract surgery pain management and the role of non-opioid alternatives. J Cataract Refract Surg. 2022;48(6):730-740.
14. Bucci FA Jr, Michalek B, Fulet AT. Comparison of the frequency of use of a pupil expansion device with and without an intracameral phenylephrine and ketorolac injection 1%/0.3% at the time of routine cataract surgery. Clin Ophthalmol. 2017;11:1039-1043.
15. Visco D. Effect of phenylephrine/ketorolac on iris fixation ring use and surgical times in patients at risk of intraoperative miosis. Clin Ophthalmol. 2018;12:301-305.
16. Walter K, Delwadia N, Coben J. Continuous intracameral phenylephrine-ketorolac irrigation for miosis prevention in femtosecond laser-assisted cataract surgery: Reduction in surgical time and iris manipulation. J Cataract Refract Surg. 2019;45(4):465-469.
17. Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74(8):477-480.

