CRST invited four surgeons to describe how they would approach the following case shared by Danson V. Muttuvelu, MD. A 71-year-old aphakic patient with pseudoexfoliation syndrome, miosis, and glaucoma was referred for secondary IOL implantation after complicated primary surgery. During the original surgery, viscodilation of the iris was performed. The lens was partially subluxated initially and became severely subluxated as the lens was rotated after hydrodissection. An OVD (Viscoat, Alcon) was instilled to tamponade the vitreous, and phacoemulsification was performed, followed by an anterior vitrectomy. The capsular bag was then removed.

KENNETH A. BECKMAN, MD, FACS
The options available to this patient include placing a secondary lens in the anterior chamber or fixating it to the iris or sclera.
Anterior chamber IOL. The patient’s history of glaucoma may steer some surgeons away from placing an anterior chamber IOL (ACIOL), yet ACIOLs are still commonly used. In a retrospective study of 182 eyes undergoing secondary IOL placement, 92 eyes (50%) received an ACIOL—the most common IOL placement in the study.1
Flexible ACIOLs are well tolerated. If the lens is positioned appropriately, it can be a successful procedure, and it is an acceptable option in the situation presented. Based on the patient’s age, I am not as worried about the long-term effects of ACIOL placement as I would be in a younger patient.
The current status of the patient’s glaucoma and overall medical status are not discussed in the case presentation. The glaucoma in this case may not be significant, and the risk that the placement of an ACIOL will worsen the glaucoma may be low. Additionally, if the patient has other medical conditions, a longer and more involved surgery may not be in their best interest.
Iris-fixated IOL. I prefer not to fixate a lens to the iris, so I would not opt for this approach.
Sutureless scleral fixation of an IOL. Scleral fixation of an IOL may be more difficult because the pupil does not dilate well. That said, several techniques for the sutureless scleral fixation of an IOL have been described. The Yamane technique, for example, can be executed quickly, but there is a learning curve with this technique. Additionally, the small pupil may add to the difficulty.
Another sutureless option is to glue the IOL haptics under a scleral flap. I have found the learning curve with this technique to be shorter than for the Yamane technique, but it may be more time consuming to execute.
Either of these techniques would be appropriate if the surgeon is comfortable and the instrumentation is available.
Sutured scleral-fixated IOL. Suturing an IOL to the sclera is an excellent option. The learning curve for the technique is short, but it can be a time-consuming procedure. Several IOLs and suture configurations may be used, depending on the surgeon’s preference. In my experience, IOL tilt is less of a problem with this approach.
A consideration with any scleral fixation technique is the risk of retinal detachment because the needle is not visible at all times. Erosion of the sclera or conjunctiva overlying the sutures or the haptic is another concern, as is infection due to potential fistula formation. Just as an ACIOL may irritate the iris and lead to inflammation, hyphema, and glaucoma, a scleral-fixated IOL may chafe the iris from the posterior side and lead to similar sequelae.
No one right approach. There are many ways to approach surgery on this patient. I cannot say that one is better than the rest. The technique with which the surgeon is most comfortable is the best option. The patient is already aphakic and out of the OR, and therefore the surgeon has time to plan.

DAVID A. GOLDMAN, MD
Unfortunately, pseudoexfoliation syndrome can increase the risks of cataract surgery because of poor pupillary dilation and zonular weakness. When dilation is borderline, I place a Malyugin Ring (MicroSurgical Technology) at the start of the case. Otherwise, the capsulorhexis may be smaller than desired, and zonular weakness may be more difficult to detect. Assuming the original surgery was performed under topical anesthesia and because the lens subluxated before phacoemulsification, I would have recommended aborting the original surgery and referring the patient for an emergent pars plana lensectomy or to an anterior segment surgeon who is comfortable using capsular hooks and performing scleral fixation of the capsular bag.
In the case described here, I would perform a dilated examination to ensure that no retained lens fragments are present. If any fragments are identified, I would perform combined surgery with the assistance of a retina colleague. I would also evaluate the optic nerve for glaucomatous damage. I would perform an endothelial cell count to ascertain if the cornea can tolerate a second procedure. If it cannot, I would discuss with the patient the possibility of combined or staged Descemet stripping automated endothelial keratoplasty combined with secondary lens insertion.
As for the surgical repair, the eye is aphakic with a poorly dilating pupil, a disrupted anterior hyaloid face, and no capsular bag. The placement of an ACIOL may increase IOP further, so I would implant a scleral-fixated posterior chamber IOL instead. My current preference is to fixate a CT Lucia (Carl Zeiss Meditec) using the Yamane technique.
I would begin by creating paired, small conjunctival peritomies and marking the eye with a toric marker to ensure symmetrical placement of the haptics. I would then inject dilute triamcinolone acetonide to detect prolapsed vitreous and perform a vitrectomy in the anterior chamber and midvitreous if necessary. If the pupil is small, I would enlarge it with a Malyugin Ring. Alternatively, if there is significant iridodonesis, one could also place iris hooks. I prefer to dock the haptics into 27-gauge needles (TSK Laboratory), as described by D. Brian Kim, MD,2 (watch a related video below) and titrate tension until the lens is well centered. If the preoperative IOP is elevated, I would consider performing a canaloplasty with the Omni Surgical System (Sight Sciences) before removing the OVD.

LUKAN MISHEV, MD
My first concerns are whether the anterior vitrectomy during the initial cataract surgery was complete and whether nuclear fragments or cortical material remains in the posterior pole. OCT imaging would be performed to determine if cystoid macular edema has developed.
For the IOL calculation, I would use a fourth-generation formula such as the Ladas super formula. The latest formulas use AI to determine the effective lens position based on a large statistical subset of eyes with different axial lengths instead of relying on lens thickness.
A Carlevale IOL (Md Tech) would be implanted and fixated with the horizontal scleral pockets technique (see below for a demonstration of the technique). This should ensure sufficient coverage of the acrylic haptics and avoid early postoperative hypotony and late conjunctival erosion. Trocars would be placed, and the vitreous base would be cleaned to locate missed cortical or nuclear remnants and prevent secondary vitreolenticular or iridolenticular aqueous flow blockage.
Postoperatively, if the IOP cannot be managed with glaucoma medications, angle surgery would be performed.

DANSON V. MUTTUVELU, MD, FEBO
The secondary IOL surgery occurred 2 months after the initial surgery. A scleral-fixated IOL was selected for this eye because the anterior chamber depth was less than 2 mm.
Stable dilation of the pupil and good visibility are crucial when working with aphakic eyes, so a 7-mm Malyugin Ring 2.0 was inserted (Figure 1). Stable dilation of the pupil also allows the surgeon to move around more freely without causing trauma to the iris. With four scrolls and eight points of fixation, the square shape of the Malyugin Ring 2.0 can evenly dilate a circular pupil and avoid the potential issues associated with iris expanders such as overstretching of the iris sphincter. The device can be useful in complex situations such as an aphakic eye or an eye with a loose capsular bag and when performing the Yamane technique.
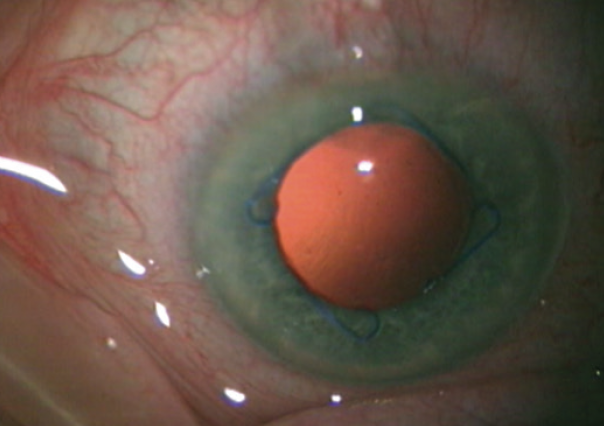
Figure 1. Pupillary dilation with a Malyugin Ring 2.0.
Infusions were delivered through a pars plana entry, and an anterior vitrectomy was performed through the pars plana. A CT Lucia IOL was implanted with the externalization technique, and the posterior haptic was passed through the main incision.3 With this technique, two thin-walled 30-gauge needles are used to make angled sclerotomies 2 mm from the limbus and 180º apart. The leading haptic of a three-piece IOL is docked into the needle lumen as the IOL exits the cartridge. Following the injection of the IOL into the anterior segment, the trailing haptic is left extending out of the main incision. On the opposite side, a needle is used to create a scleral tunnel and then externalized through the main incision to allow docking of the trailing haptic via the main incision (Figure 2). The haptics are externalized, and cautery is used to create a wider flange at the haptic tip. The haptics are then settled back into the sclerotomies for stability by burying the flanged tips.
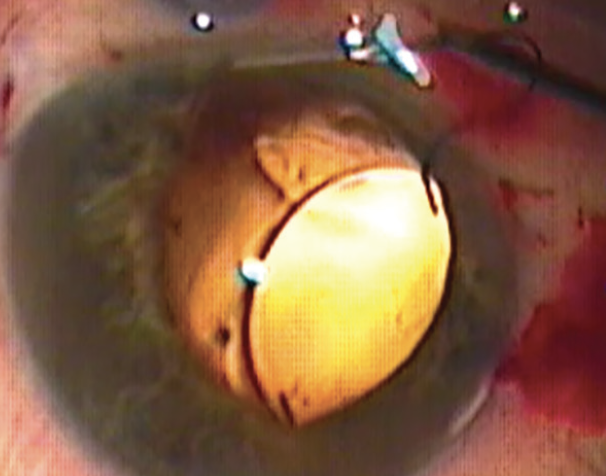
Figure 2. Extraocular docking of the trailing haptic via the main incision.
Figures courtesy of Danson V. Muttuvelu, MD, FEBO
Postoperatively, the patient’s UCVA was 6/7, and BCVA was 7/7 with +0.50 D of sphere. The patient was happy and discontinued follow-up after 6 months.
1. Vounotrypidis E, Schuster I, Mackert MJ, Kook D, Priglinger S, Wolf A. Secondary intraocular lens implantation: a large retrospective analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257(1):125-134.
2. Kim DB. The next big thing in scleral haptic fixation. Cataract & Refractive Surgery Today. October 2017. Accessed March 16, 2021. https://crstoday.com/articles/2017-oct/the-next-big-thing-in-scleral-haptic-fixation
3. Ifantides C, Naids SM, Muttuvelu DV, Mian SI, Christopher KL. A modified flanged intrascleral intraocular lens fixation technique using an externalized needle for haptic docking. Clin Ophthalmol. 2021;15:2047-2050.