The standard of care for a clinically significant cataract is the surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). Since the invention of the IOL, different biomaterials with different optical and mechanical properties have been utilized to produce IOLs. AcrySof® by Alcon is a widely used, proven IOL platform with more than 125 million implantations worldwide over the past 30 years.1 Recently, Neda Shamie, MD, moderated a discussion with J. Morgan Micheletti, MD; Brian Shafer, MD; and Liliana Werner, MD, PhD, to review the latest innovation in IOL technology from Alcon, the Clareon® IOL.
Dr. Shamie: Cataract surgery is the center of the ophthalmic surgical practice, and the technology we rely on is an important topic of conversation among surgeons and with patients. Lens optical designs have improved, presbyopia-correcting lenses have become more commonplace, and premium lenses add a new level of interest and complexity into our practices. As surgeons, we need to be thoughtful about what lenses we choose to offer our patients. This is an extension of our surgical expertise and one that we need to familiarize ourselves with. The AcrySof® IOL platform has been a huge part of my practice and that of cataract surgeons around the world, and for good reason.1
Attributes of Acrysof® IQ monofocal iol
Dr. Shamie: We will start with an in-depth look regarding what qualities we look for in a monofocal IOL, such as the AcrySof® IQ monofocal IOL (Alcon).
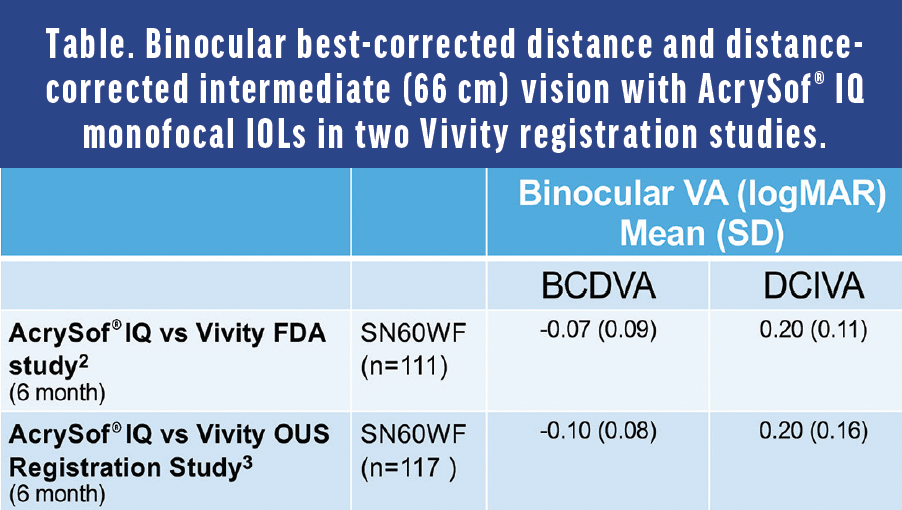
Dr. Micheletti: Monofocal IOLs should provide excellent distance vision. I often assess the defocus curve because it provides information about where the vision with an IOL will land at different amounts of defocus or simulated viewing distances. In general, a defocus curve is made by starting the patient at the best-corrected distance visual acuity, then defocusing by adding more minus to simulate moving the distance target closer. In two large AcrySof® IQ Vivity registration studies, the AcrySof® IQ monofocal IOL, used as a control, showed its binocular defocus curve plateaus (≥20/20) around zero diopters on either side (±0.50 D) (Figure 1).2,3 This translates to a broad “landing zone,” which may be related to the AcrySof® depth of focus, a concept rarely discussed with monofocal IOLs until recently. In fact, in addition to confirming its excellent distance vision (≥20/20), an incidental finding in the Vivity studies showed that the AcrySof® IQ monofocal IOL provided functional intermediate vision. At 66 cm, patients were seeing about 20/32 (Table)2,3, which is quite remarkable.
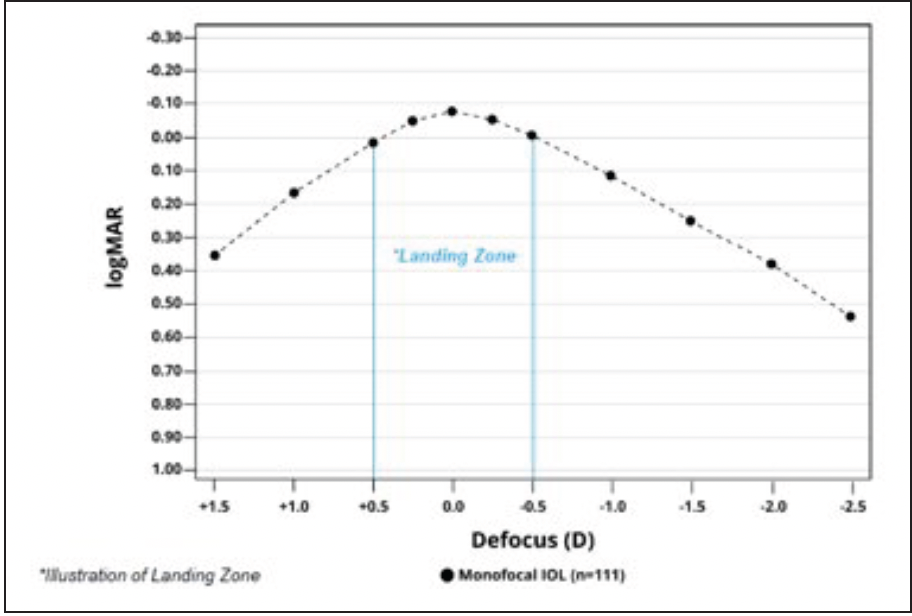
Figure 1. Binocular defocus curve with AcrySof® IQ monofocal IOL in AcrySof® IQ Vivity FDA registration study (6 months post-implantation).2
Dr. Shamie: In my experience, there is forgiveness to IOL power targeting accuracy with the AcrySof® IQ, as you said. If the refractive outcome is between plus or minus 0.50 D, the average patient still achieves 20/20 at distance and may have some more depth of focus if you err a little on the minus side.3 Our cataract patients are becoming more and more savvy on digital technology. Functional intermediate vision with a monofocal IOL like AcrySof® IQ is a bonus. So, it is important to look at the defocus curves and the intermediate vision for monofocal lenses and not just for presbyopia-correcting lenses.
Dr. Shafer: IOL mechanical stability, mainly affected by the haptic design and in part by the biomaterial, is another critical performance attribute that affects the clinical outcomes of cataract surgery. The STABLEFORCE Haptics (Alcon) of the AcrySof® IQ contain a planar haptics design with a flexible hinge.4 These haptics provide for low axial displacement, decentration, and tilt,5 which contributes to refractive accuracy and stability. Rotational stability of a toric lens is crucial for astigmatism correction, and multiple studies have shown that AcrySof® toric IOLs are rotationally superior to lenses such as the original TECNIS toric and enVista toric IOLs.6-10
Dr. Werner: Lens edge design and biomaterial play an important role in posterior capsular opacification (PCO) formation, a common, yet non-serious, adverse event after IOL implantation that can affect vision to the point of needing a Nd:YAG capsulotomy. The AcrySof® IOL, made of a biocompatible hydrophobic acrylic material with adhesive properties, incorporates a posterior square edge, which is designed to help prevent residual lens epithelial cells at the equatorial region of the capsular bag from growing along the posterior capsule, behind the optic.4 I participated in studies using pseudophakic cadaver eyes and immunochemistry to compare the AcrySof® lens with other IOL materials. We demonstrated that there is more binding of fibronectin to the surface of the AcrySof® lens.11,12 This is important because fibronectin protein mediates adhesion between the optic and the capsular bag, and this early attachment of the lens to the bag has the potential to reduce the incidence of PCO.
Dr. Micheletti: Real-world clinical data show low Nd:YAG capsulotomy rates for AcrySof® IOLs versus hydrophilic and other hydrophobic IOLs.4,13,14 Having IOLs with low incidence of PCO that requires YAG can enhance patients' overall experience.
It is also noteworthy that the refractive index of the AcrySof® material is high at 1.55, which allows for implantation of the lens with a fully usable 6 mm optic through a small incision from low to high diopter lenses.4
Dr. Shamie: We have been very successful with the AcrySof® IOL platform for the last 30 years and it has, as mentioned, offered excellent attributes, including high-quality distance and functional intermediate vision, stable postoperative refractive outcomes due to the high axial and rotational stability, and relatively low incidence of PCO requiring Nd:YAG capsulotomy. But with any platform, there is room for improvement, and if there’s one concern about the AcrySof® platform that some surgeons have reported, it is the finding of glistenings. Although, as we know, glistening formation is not unique to AcrySof® IOLs and its clinical relevance as far as impacting visual function is not confirmed15, we sometimes observe it during follow-up visits at the slit lamp, with concern that some patients might come back years later with visual complaints.
In response to surgeon feedback, Alcon improved the AcrySof® manufacturing process and showed an 87% reduction in glistening formation in IOLs manufactured after 2012.16 Alcon responded further to our demands with the new Clareon® platform, which utilizes a new biomaterial that takes advantage of the characteristics that gave the AcrySof® platform the leading position among IOLs for 30 years. The Clareon® IOL meets physician demands for not only function and performance but also improved optical clarity.
an advanced IOL built upon the legacy of AcrySof® platform
Dr. Shamie: Let’s talk about how the Clareon® IOL is similar to, and how it has improved upon, the AcrySof® IQ IOL. Dr. Werner, will you talk about the materials used to manufacture the Clareon®?
Dr. Werner: The Clareon® IOL is made of an advanced hydrophobic acrylic biomaterial incorporating HEMA, or hydroxyethylmethacrylate.17,18 HEMA uniformly distributes water throughout the polymer matrix, increasing the water content to 1.5% as compared to 0.4% in the AcrySof®. This increase in water content seems small, but it significantly contributes to the improved optical clarity characteristics of the lens, including reducing glistenings.18 Additionally, Alcon incorporated multiple manufacturing advancements19,20 including improvements on equipment, laboratory methods, quality control, and processes such as molding and milling. All these play a role in increasing the optical clarity of the lens and contribute to a smooth lens surface and precision edge design.17,20
Dr. Micheletti: The Clareon® IOL is built with many of the same positive design attributes as the AcrySof® IQ IOL. Clareon® IOLs maintain the 1.55 high refractive index, an important characteristic that allows a thinner overall lens to achieve the same refractive power and enable the same full 6-mm usable optic design (Figure 2) to be delivered through a small incision.17
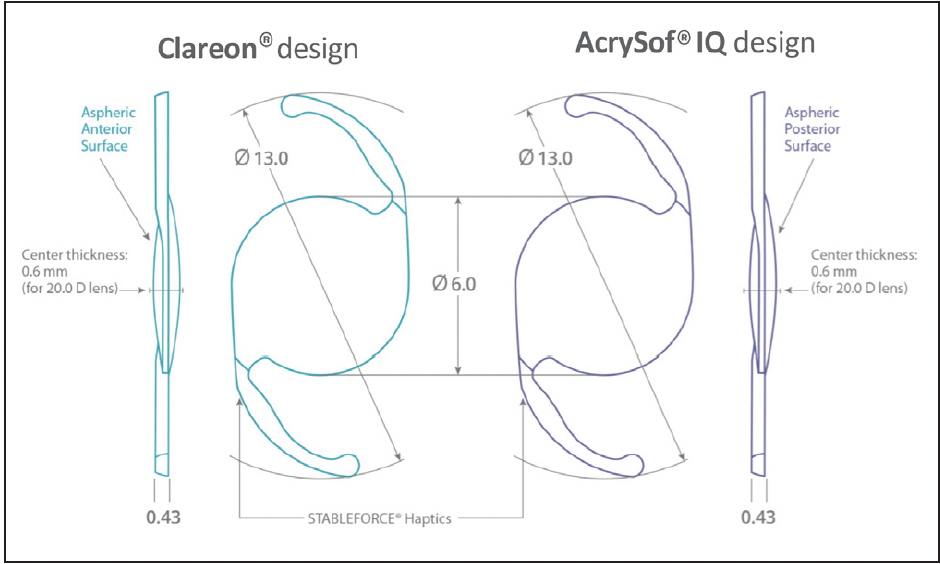
Figure 2. Comparison of Clareon® to AcrySof® IQ monofocal IOL design.4,17
Dr. Werner: In order to maintain a constant central thickness or reduce the lens volume across different dioptric powers to allow small-incision IOL delivery, manufacturers of other IOLs made with low refractive index materials incorporated peripheral non-imaging elements to the IOLs. This results in a smaller usable optic (typically around 5 mm). These non-imaging elements can increase positive dysphotopsias.21 The Clareon® IOL does not have any of these elements.
Dr. Micheletti: Utilization of the full 6-mm optic compared to only 5 mm, as you just described, may also contribute to good visual performance in patients with large pupils in mesopic or dim light conditions.2,3
Dr. Shafer: Another feature that carried over from the AcrySof® IQ IOL to the Clareon® IOL to help provide high quality of vision is the optical design with a -0.2 mm asphericity to compensate the natural positive corneal spherical aberration. All new IOLs go through a series of tests, including optical tests, and must pass several international standards. Modulation transfer function (MTF) measures the ability of a lens to transfer an object’s contrast to its image and is reported as a ratio of image contrast to object contrast. The MTF of the Clareon® monofocal IOL far exceeds the international standards.22 Equivalency on MTF was also demonstrated for Clareon® IOL and AcrySof® IQ IOL for both 3-mm and 5-mm apertures, indicating that the Clareon® monofocal IOL has equivalent optical performance to the AcrySof® IQ monofocal IOL (Figure 3).22
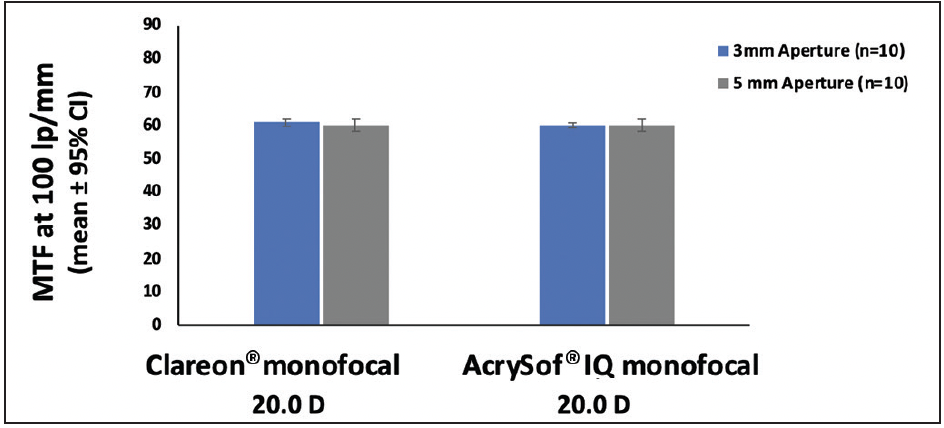
Figure 3. Comparison of MTF using the 0.2 µm SA modified ISO model with 550 nm green light (n=10, 20.0 D)
STABLEFORCE haptics and Mechanical Stability
Dr. Shamie: Lens design, specifically haptics, play a key role in lens performance. The Clareon® IOL kept the same proven and effective haptic design as the AcrySof®, and I think we can all agree that haptic design is very important to refractive outcomes. Dr. Micheletti, could you comment on STABLEFORCE haptics?
Dr. Micheletti: When we implant a lens, we want it to stay in place and end up at the calculated refractive power. STABLEFORCE haptics allow contraction in varying bag sizes without significant axial displacement, decentering, or tilting of the IOL which could result in a change in the refractive correction. An in vitro compression study showed Clareon® and AcrySof® IQ IOLs with STABLEFORCE haptics had the lowest axial shift (Figure 4), comparable decentration, and lower tilt versus some other tested IOLs.5
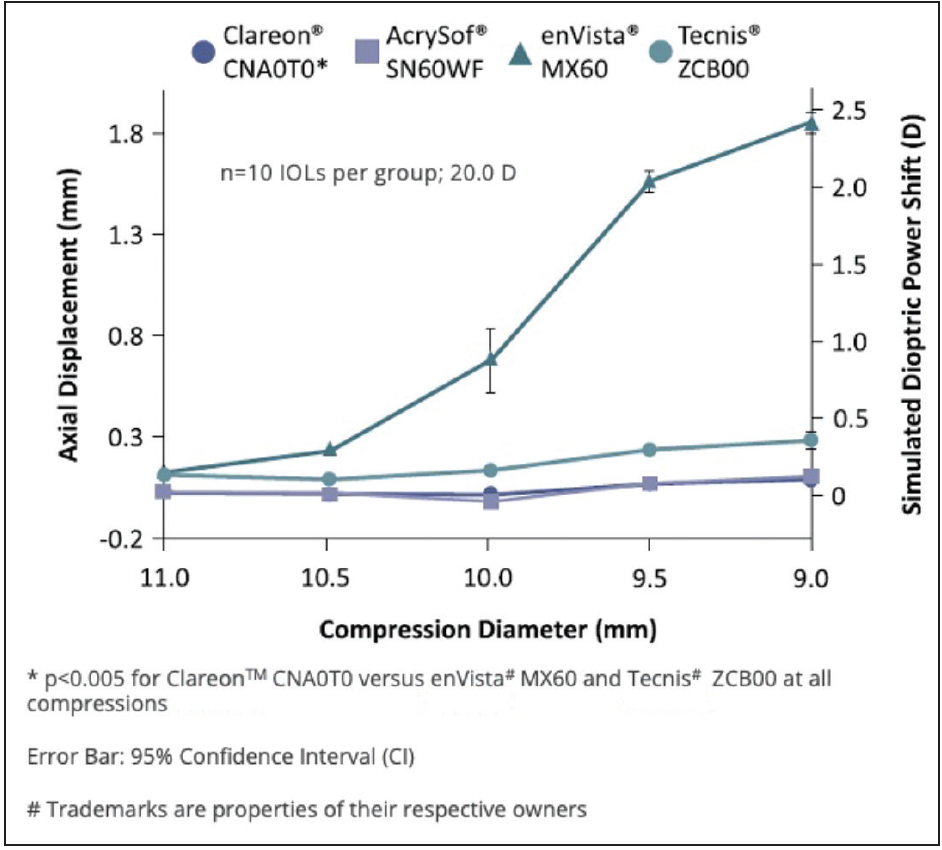
Figure 4. In vitro comparison of axial displacement and simulated dioptric power shift among different IOLs.
Dr. Werner: In a preclinical study, we tested mechanical stability in vivo using a rabbit model.23 In 15 rabbits, we compared the Clareon® IOL with the AcrySof® IQ and evaluated multiple parameters including measurement with ultrasound biomicroscopy (UBM) of the anterior chamber depth (ACD) at 1 and 4 weeks postoperatively (Figure 5). We found that the axial displacement in terms of changes of ACD measurements were similar between the AcrySof® eyes and the Clareon® eyes, and both were very stable over time.23
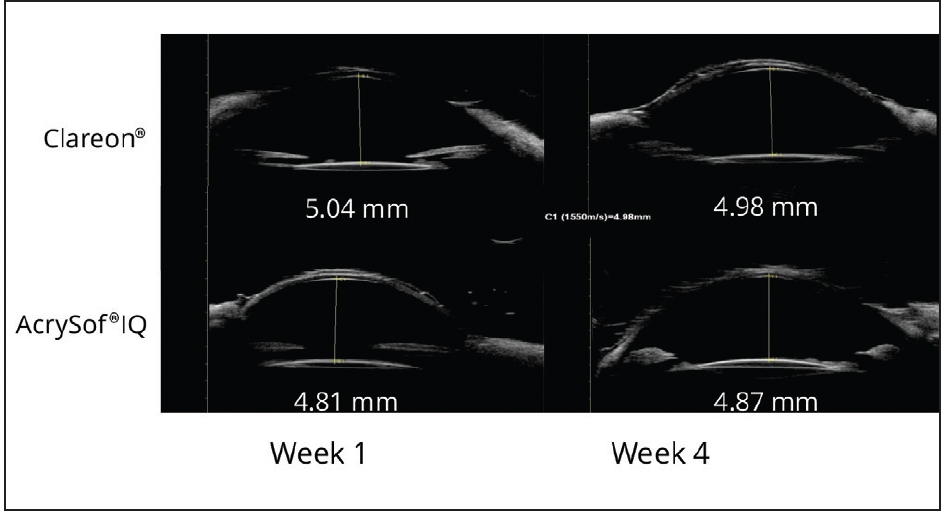
Figure 5. UBM scans of two rabbit eyes implanted with Clareon® and AcrySof® IQ, taken at 1 and 4 weeks postoperatively.23
Another study published by Oliver Findl et al in Austria compared the same two lenses contralaterally implanted.24 They followed 80 eyes of 40 patients up to 6 months and measured the ACD. They could not demonstrate any clinically relevant differences in ACD between two groups. These findings from their clinical study correlate well with the preclinical studies.5,23
Dr. Shafer: It is critical that a patient’s refractive outcome remains stable over time. We have multiple clinical studies reporting that Clareon® IOL implantation in cataract patients is safe and effective with stable refractive and visual outcomes from postop day 1 to month 12.25-27
Visual Outcomes at year 1 for 350 patients over 16 sites in the FDA registration study showed 99.7% of participants achieved monocular CDVA ≤0.3 logMAR, exceeding the historical safety and performance endpoint rate with a mean monocular distance-corrected visual acuity at -0.05 logMAR.25 Mean manifest refraction spherical equivalent (MRSE) of these patients also remained stable from week 1 to year 1 with 0.00 D median MRSE at all postop visits (Figure 6).
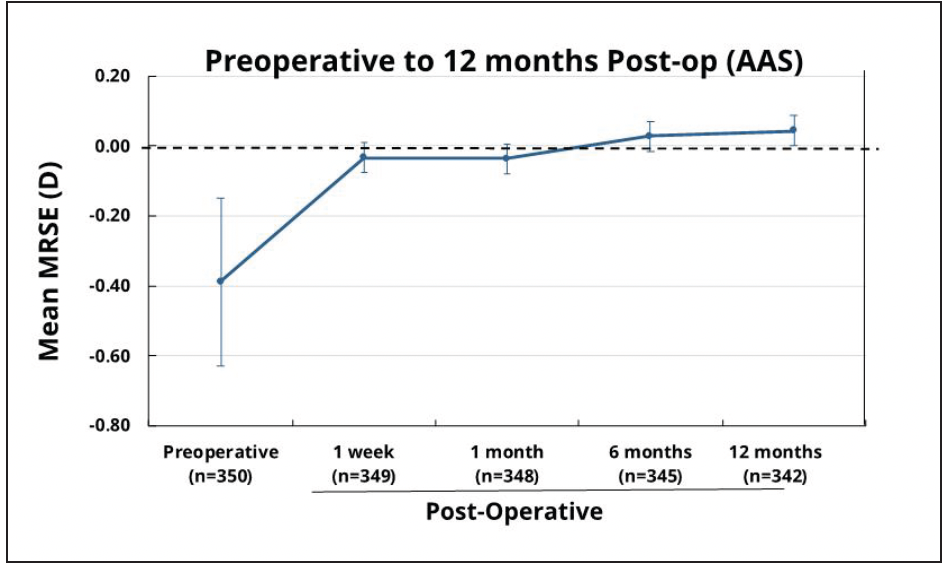
Figure 6. Mean MRSE from pre-operative to 12 months postoperative in subjects who received Clareon® monofocal IOL. Error bars represent 95% CIs.
The FDA study also evaluated the rotational stability of the Clareon® IOL on a subset of 141 patients over six sites.28 Masked graders looked at IOL axis at postop ≤ hour 1 (baseline), day 1, week 1, and month 1, and at month 6 to determine rotational stability; 95.3% and 93% of patients had less than 5° of rotation at day 1 and at month 6, respectively. From a rotational standpoint, these lenses are very stable in the eye.
Dr. Shamie: The refractive and rotational stability data from the FDA registration study indicate excellent mechanical stability of Clareon® IOLs. This information is going to be even more important for us when advanced technology IOLs are applied to the Clareon® platform. As we know, the demand for refractive accuracy and stability for these advanced technology IOLs is even higher.
Precision Edge Design
Dr. Shamie: There is a lot more that goes into a lens design than we, as surgeons, may think about. And one factor is obviously the edge design, as it may impact the PCO formation and photic phenomena, such as glare. Dr. Werner, can you comment on this aspect of the Clareon® IOL?
Dr. Werner: The edge profile of a lens potentially impacts the amount of light that is transmitted or reflected away from the main beam image. Studies using ray tracing simulations and optical bench tests help us understand the photic phenomena incurred on various IOLs when off-axis light shines on them. The Clareon® IOL has two characteristics that specifically take this into consideration. The first is that the Clareon® IOL has a fully usable 6 mm optic from low to high diopter lenses without requiring any non-imaging peripheral elements, such as flanges that are used in some other IOLs. The second characteristic is, unlike some IOLs with a square truncated optic edge design,21 the Clareon® IOL has a square edge on the posterior optic surface of the lens designed to help reduce PCO.17,29 The precision edge design also incorporates a proprietary curvature of the lateral wall of the lens optic (Figure 7), designed to help decrease transmitted or reflected light.17,21
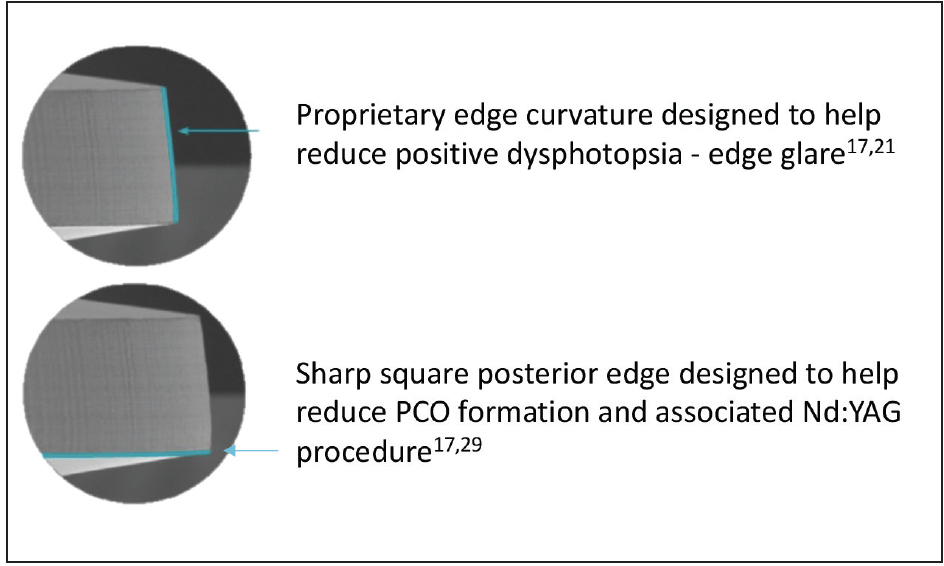
Figure 7. Edge Design shown under scanning electron microscopy.
Studies using the two above-mentioned methods showed that the values of transmitted or reflected light with the Clareon® IOL remained quite stable. Compared to Clareon®, with increasing angles of off-axis illumination, some lenses had significantly higher levels of glare (Figure 8).21 Of course, these are in vitro results, and clinical studies, especially under dim light or night driving, are necessary to confirm the clinical relevance of such findings.
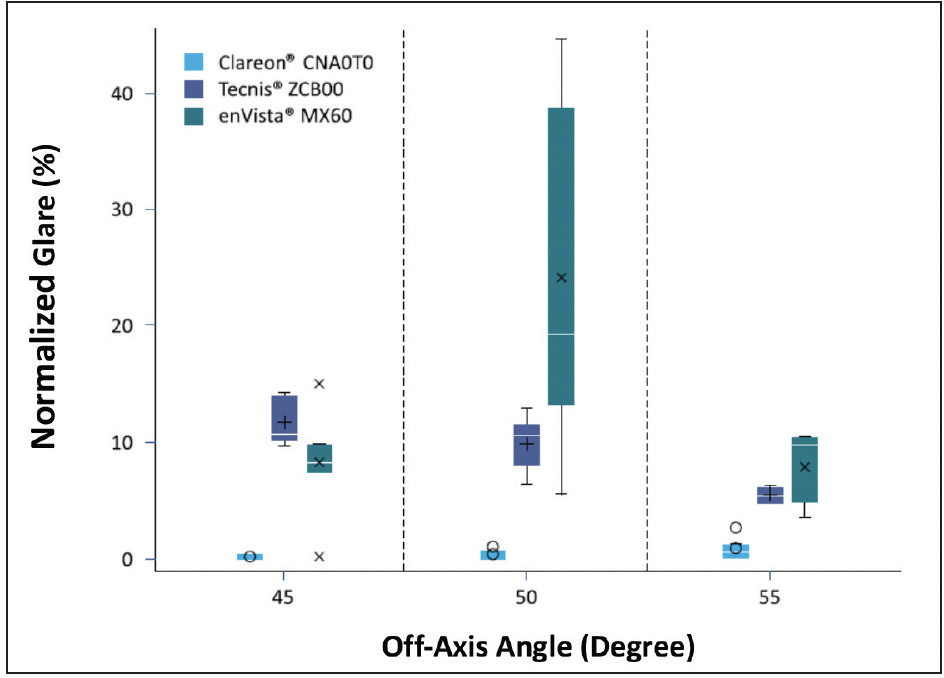
Figure 8. Statistical comparison of in vitro glare evaluation results for 45°, 50°, and 55° off-axis illumination for Clareon® CNA0T0 (Alcon), Tecnis ZCB00 (Johnson & Johnson Vision), and enVista MX60 (Bausch + Lomb) (all 25 D).
Clareon® maintains the same characteristics of the AcrySof® lens with a square edge on the posterior optic surface, which was designed to help reduce PCO formation. In the rabbit study23 I mentioned before, as well as a study with a human capsular bag model30, similar PCO formation with AcrySof® and Clareon® IOLs were reported.
Dr. Shafer: In the Clareon® IOL FDA registration study,25 the rate of PCO reported was 5.4% (19/350), and the rate of Nd:YAG capsulotomy was 4.6% (16/350). Although no direct comparison with AcrySof® was done in this study, the data suggest the rates are somewhat higher than the range reported for AcrySof® IOLs. However, 6 of 16 subjects who underwent Nd:YAG for Clareon® were from a single site where a total of 20 subjects were enrolled. This is very important context that helps with the interpretation of the results for PCO requiring Nd:YAG. The average rate of Nd:YAG capsulotomy among the remaining sites was 3%.
Dr. Shamie: Several other studies27,31,32 with similar follow-up periods showed lower Nd:YAG rates than the FDA registration study. Larger sized clinical studies with longer follow up will help to understand the rates of PCO formation and Nd:YAG capsulotomy.
Optical Clarity
Dr. Shamie: One motivation behind Alcon’s development of Clareon® IOL is to further improve optical clarity, which is an important parameter in the quality assessment of IOLs. Optical clarity not only includes glistenings, but also surface haze and subsurface nanoglistenings (SSNG). Let’s break it down and have a better understanding of the potential causes and manifestations, as well as the study data found with the Clareon® IOL.
Dr. Micheletti: Surface haze is related to the nanosized rough surface texture associated with the manufacturing process. It can be observed under the slit lamp using oblique light immediately after surgery and appears as a grainy matte texture on the IOL surface.
Dr. Shafer: Both glistenings and SSNG can gradually develop after IOL implantation, more often with hydrophobic acrylic materials. They are caused by hydration-related phase separation. Glistenings are fluid-filled microvacuoles that range in size from 1-20 μm, located throughout the thickness of the IOL optic. SSNGs are nanovacuoles that are smaller than 200 nm in size and are situated 120 μm or less from the IOL surface.
Dr. Werner: We performed an in vitro study evaluating the optical clarity characteristics of the Clareon® IOL comparing them to the Tecnis (Johnson & Johnson Vision) and enVista (Bausch + Lomb) IOLs.18 We assessed surface haze under a slit lamp and took photographs to measure pixel haze intensity, which correlates with IOL surface roughness. We also measured surface roughness directly using atomic force microscopy. The Clareon® IOL had a smoother surface in comparison to the other IOLs (P<0.001).
Next, we evaluated SSNG. The literature shows that SSNG increase over time. We artificially aged the lenses to simulate up to 10 years of implantation. Using Scheimpflug photography, we then measured back light scattering associated with SSNG. The Clareon® IOL has among the lowest levels of back light scattering compared to other hydrophobic acrylic lenses on the market (Figure 9).

Figure 9. SSNG – In Vitro Scheimpflug imaging of IOLs with 10-year accelerated aging showing low peak surface scatter intensity with the Clareon® IOL.
In this same study, we induced glistenings formation by putting the IOL in a solution and changing the temperature from a high to a low temperature, for example, from 45°C to body temperature. We photographed the lenses with a camera coupled to a microscope with high magnification and digitally analyzed the photographs to quantify any microvacuole formation corresponding to glistenings. Glistenings formation associated with the Clareon® IOL was lower than with TECNIS lenses and not significantly different from the results of the enVista IOL, which is considered glistening free (Figure 10).33
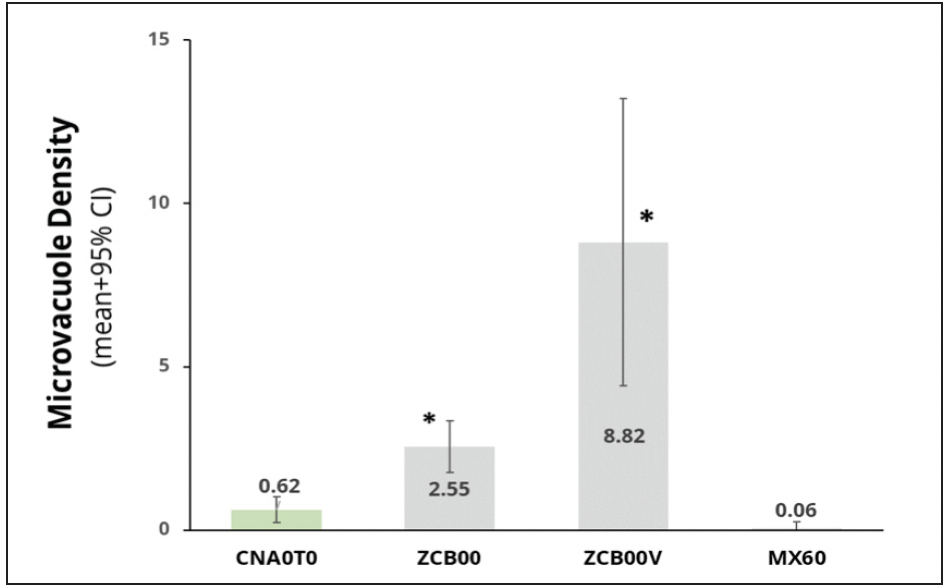
Figure 10. The in vitro study showed the Clareon® IOL had low Microvacuole Density, correlating to few glistening formations.
The modified Miyata scale is a widely used method to quantify and compare glistenings in clinical studies. Glistenings are scored from zero to three according to the number of microvacuoles seen per square millimeter (MV/mm2). Grade 0 is 0-25 MV/mm2, grade 1 is 26-75 MV/mm2, grade 2 is 76-150 MV/mm2, and grade 3 is more than 150 MV/mm2. According to the modified Miyata scale, the Clareon® IOL is a grade zero, supporting the conclusion that the Clareon® IOL is glistening free.*
*Defined as modified Miyata scale 0 or <25 MV/mm2
Dr. Shafer: It’s great hearing about the benchwork that goes into ensuring that the optical clarity is on par, and it’s amazing to have the clinical data to support it. A recently published Japanese clinical study followed patients implanted with a Clareon® IOL in one eye and an AcrySof® IQ in the contralateral eye for up to 7 years.34 Measurement of surface light scattering, corresponding to SSNG, was significantly lower in the Clareon® eyes (P<0.001). Although the SSNG measurements increased in the AcrySof® eyes, no statistically significant difference was found in the corrected-distance visual acuities and contrast sensitivity of those eyes. We should note that the AcrySof® IOLs used in this specific study were manufactured before the manufacturing process improvements in 2012.
In the FDA registration study for the Clareon® IOL, 350 eyes were followed across 1,852 postoperative visits over 1 year. There were no IOL observations for surface haze, SSNG, or glistenings.25
Dr. Micheletti: There are additional clinical data regarding clarity characteristics of the Clareon® IOL, including long-term data. Dr. Oshika and colleagues in Japan performed a clinical study evaluating 110 eyes with the Clareon® IOL for 1 year with a subset of 20 eyes followed for 9 years. In this study, glistenings and surface light scattering corresponding to subsurface nanoglistenings were not seen in any of the eyes.26 In another study, Oshika et al reported 100% grade 0 glistenings after a 12-month follow-up (n = 384 eyes) in a multi-center case evaluation study.27
Dr. Shamie: Adding to all this data supporting the glistening-free nature of the Clareon® material, a prospective multinational single-arm study recently reported at ASCRS showed all Clareon® IOL implanted eyes (n = 365) had grade zero glistenings at the 3-year mark.35 And finally, another study by Dr. Stanojcic and colleagues looked at Clareon® IOL implants at the 1-year mark, and again showed the same grade 0 results for glistenings.32
Conclusion
Dr. Shamie: We discussed laboratory data and clinical data with long-term results that show the benefits of the Clareon® IOL. Now suppose, as your colleague, I say that I do not use AcrySof® because of the glistenings. Tell me why I should consider the Clareon® IOL.
Dr. Micheletti: Look at the data. The question around glistenings was addressed by the clinical data collected out to as far as 9 years across multiple studies showing the glistening-free* characteristics of the Clareon® IOL. This lens has been in development for a long time, is well thought out, and has the science and research to back it. Given the study findings and my own clinical experience, I feel very confident telling another surgeon to try it.
Dr. Werner: The improved optical clarity characteristics are undeniable, and everything in the Clareon® design and manufacturing process improvement has a reason; it is not just to be beautiful, but also offers the function to improve patients’ vision.
Dr. Shafer: For my hesitant peers, I encourage you to reflect on what criteria you look for in an IOL. If the Clareon® IOL doesn’t meet your criteria, continue using the lens most comfortable in your hands. We are all on one team: clinicians, scientists, industry, and the patients, sharing a common goal of improving vision. With the IOLs available, and the new Clareon® IOL, we can achieve that goal together.
Important Product Information - Clareon® Family of IOLs
CAUTION: Federal law restricts these devices to sale by or on the order of a physician.
INDICATION: The family of Clareon® intraocular lenses (IOLs) includes the Clareon® Aspheric Hydrophobic Acrylic and Clareon® Aspheric Toric IOLs, the Clareon® PanOptix® Trifocal Hydrophobic IOL, Clareon® PanOptix® Toric, Clareon® Vivity™ Extended Vision Hydrophobic Posterior Chamber IOL and Clareon® Vivity™ Toric IOLs. Each of these IOLs is indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the Clareon® Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The Clareon® PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The Clareon® Vivity™ lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag.
WARNINGS/PRECAUTIONS:
General cautions for all Clareon® IOLs:
Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting any IOL in a patient with any of the conditions described in the Directions for Use that accompany each IOL. Physicians should target emmetropia, and ensure that IOL centration is achieved.
For the Clareon® Aspheric Toric, PanOptix® Toric and Vivity™ Toric IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
For the Clareon® PanOptix® IOL, some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos or starbursts, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs.
For the Clareon® Vivity™ IOL, most patients implanted with the Vivity™ IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the Clareon® Vivity™ IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the parent AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with these IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
AcrySof® Single-Piece Monofocal Intraocular Lenses Important Product Information
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
INDICATIONS: AcrySof® single-piece monofocal intraocular lenses (IOLs) include the AcrySof® IQ Aspheric Natural IOL (Model SN60WF), AcrySof® UV-Absorbing Aspheric IOL (Model SA60WF), AcrySof® Natural IOL (Model SN60AT) and AcrySof® IOL (Model SA60AT). Each IOL is indicated for visual correction of aphakia in adult patients following cataract surgery. These IOLs are intended for replacement in the capsular bag.
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. Toric IOLs should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation. All viscoelastics should be removed from both the anterior and posterior sides of the lens; residual viscoelastics may allow the lens to rotate. Optical theory suggests that high astigmatic patients (i.e., > 2.5 D) may experience spatial distortions. Possible toric IOL related factors may include residual cylindrical error or axis misalignments. Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon for this product informing them of possible risks and benefits associated with the AcrySof® IQ Toric Cylinder Power IOLs. Studies have shown that color vision discrimination is not adversely affected in individuals with the AcrySof® Natural IOL and normal color vision. The effect on vision of the AcrySof® Natural IOL in subjects with hereditary color vision defects and acquired color vision defects secondary to ocular disease (e.g., glaucoma, diabetic retinopathy, chronic uveitis, and other retinal or optic nerve diseases) has not been studied. Do not resterilize; do not store over 45° C; use only sterile irrigating solutions such as BSS® or BSS PLUS® Sterile Intraocular Irrigating Solutions.
ATTENTION: Reference the Directions for Use labeling for a complete listing of indications, warnings and precautions.
US-CLI-2200219
1. Alcon Data on File (2020).
2. AcrySof IQ Vivity IOL DFU.
3. Bala et al. Multi-country clinical outcomes of a new nondiffractive presbyopia-correcting. J Cataract Refract Surg. 2022;48:136-143.
4. AcrySof IQ DFU.
5. Lane et al. Evaluation of intraocular lens mechanical stability. J Cataract Refract Surg. 2019;45:501-506.
6. Lee B, Chang D. Comparison of the rotational stability of two toric IOL in 1273 consecutive eyes. Ophthalmology. 2018;125(9):1325-1331.
7. Potvin et al. Toric intraocular lens orientation and residual refractive astigmatism: an analysis. Clinical Ophthalmol. 2016;10:1829-1836.
8. Oshika et al. Comparison of incidence of repositioning surgery to correct misalignment with three toric intraocular lenses. Eur. J. Ophthalmol. 2020 30(4):680-684. Epub 2019 Mar 6.
9. Kramer et al. Rotation Characteristics of Three Toric Monofocal Intraocular Lenses. Clinical Ophthalmol. 2020:14 4379–4384.
10. Kramer et al. Real-World Incidence of Monofocal Toric IOL Repositioning: Analysis of Patients in the American Academy of Ophthalmology IRIS Registry. J Cataract Refract Surg. 2022;48:298-303.
11. Linnola RJ, Werner L, Pandey SK, Escobar-Gomez M, Znoiko SL, Apple DJ. Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materialsin pseudophakic human autopsy eyes. Part 1: histological sections. J Cataract Refract Surg. 2000;26(12):1792–1806.
12. Ong et al. Fibronectin adhesive properties of various intraocular lens materials. Alcon Laboratories, Fort Worth, TX, USA. ARVO 2013.
13. Ursell et al. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: a real-world evidence study of 20,763 eyes. Eye (2020). 34:960–968.
14. Horn et al. Incidence of YAG Due to PCO Following Intraocular Lens Implantation: an Analysis of AAO IRIS Registry. Presented at: AAO 2019, San Francisco.
15. Werner L. Glistenings and surface light scattering in intraocular lenses. J Cataract Refract Surg. 2010; 36:1398–1420.
16. Thomes BT, Callaghan TA. Evaluation of in vitro glistening formation in hydrophobic acrylic intraocular lenses. Clinical Ophthalmology. 2013:7 1529–1534.
17. Clareon DFU.
18. Werner et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019; 45:1490–1497.
19. Wang et al. Quantification of the in vitro predisposition to glistening formation in one manufacturer’s acrylic intraocular lenses made in different decades. Ophthalmol Ther. 2021; https://doi.org/10.1007/s40123-020-00329-8.
20. Alcon Data on File (2018).
21. Das et al. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena: Comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;425:219-227.
22. Alcon Data on File (2022).
23. Werner et al. In vivo evaluation of a new hydrophobic acrylic intraocular lens in the rabbit model. J Cataract Refract Surg. 2018; 44:1497–1502.
24. Ullrich M, Ruiss M, Hienert J, et al. Anterior chamber depth variability between 2 hydrophobic acrylic 1-piece intraocular lenses: randomized trial. J Cataract Refract Surg. 2021 Nov 1;47(11):1460-1465. doi: 10.1097/j.jcrs.0000000000000668.
25. Lehmann et al. Effectiveness and safety of the Clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clinical Ophthalmology. 2018 15, 1647.
26. Oshika T et al. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46:682-687.
27. Oshika T, Sasaki N. One-year multicenter evaluation of a new hydrophobic acrylic intraocular lens with hydroxyethyl methacrylate in automated preloaded delivery system. J Cataract Refract Surg. 2022;48:275-279.
28. Walters TR, Lehmann R, Moyes A, et al. Rotational stability of the Clareon monofocal aspheric hydrophobic acrylic intraocular 24 lens 6 months after implantation. Clin Ophthalmol. 2022;16:401-409.
29. Von Tress et al. A meta-analysis of Nd:YAG capsulotomy rates for two hydrophobic intraocular lens materials. Clin Ophthalmol. 2018;12:1125-1136.
30. Hillenmayer A, Wertheimer CM, Kassumeh S, et al. Evaluation of posterior capsule opacification of the Alcon Clareon IOL vs the Alcon Acrysof IOL using a human capsular bag model. BMC Ophthalmol. 2020;20(1):77. doi:10.1186/s12886-020-01349-5.
31. Alcon Data on File (2016).
32. Stanojcic N, OʼBrart D, Hull C, Wagh V, Azan E, Bhogal M, Robbie S, Li JO. Visual and Refractive Outcomes and Glistenings Occurrence After Implantation of 2 Monofocal, Aspheric, Hydrophobic Acrylic IOLs. J Cataract Refract Surg. 2020;46:986-994.
33. enVista DFU.
34. Kinoshita et al. Surface light scattering from 1-piece hydrophobic acrylic intraocular lenses with hydroxyethyl methacrylate: contralateral observation for 7 years. J Cataract Refract Surg 2021; 47:702–70.
35. Nuijts et al. Multinational Evaluation of a New Aspheric Hydrophobic Monofocal Intraocular Lens 3 Years after Implantation. ASCRS 2022.







