CASE PRESENTATION
A 50-year-old man presents with decreased vision after bilateral cataract surgery with trifocal IOL implantation. He states that, immediately after surgery, he felt as if he were looking through a dark orange tint.
On examination, the patient’s UCVA is 20/80 OU. The IOP is 15 mm Hg OD and 14 mm Hg OS with applanation tonometry. A slit-lamp examination reveals an IOL haptic in the sulcus of each eye (Figure 1) and significant iris transillumination defects in the left eye. A dilated fundus examination finds healthy optic nerves and no retinal pathology in either eye. The topography and biometry measurements are shown in Figures 2 and 3, respectively.
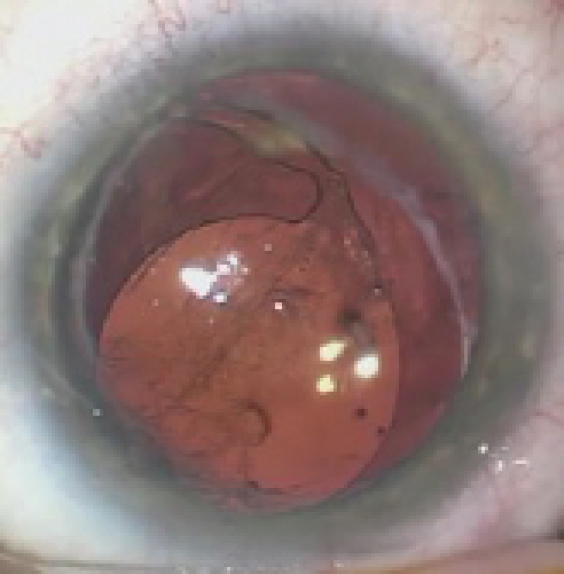
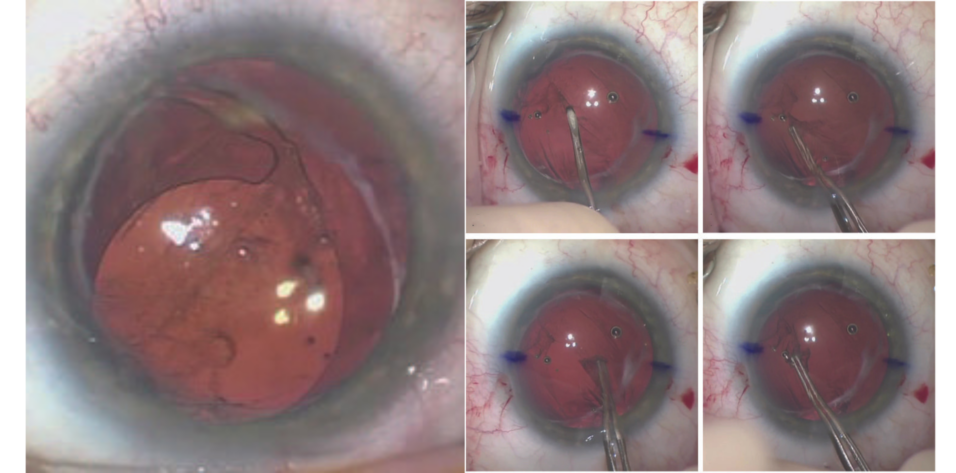
Figure 1. The IOL in the right eye has dislocated inferiorly. The superior haptic appears to be in the capsular bag and the inferior haptic in the sulcus.
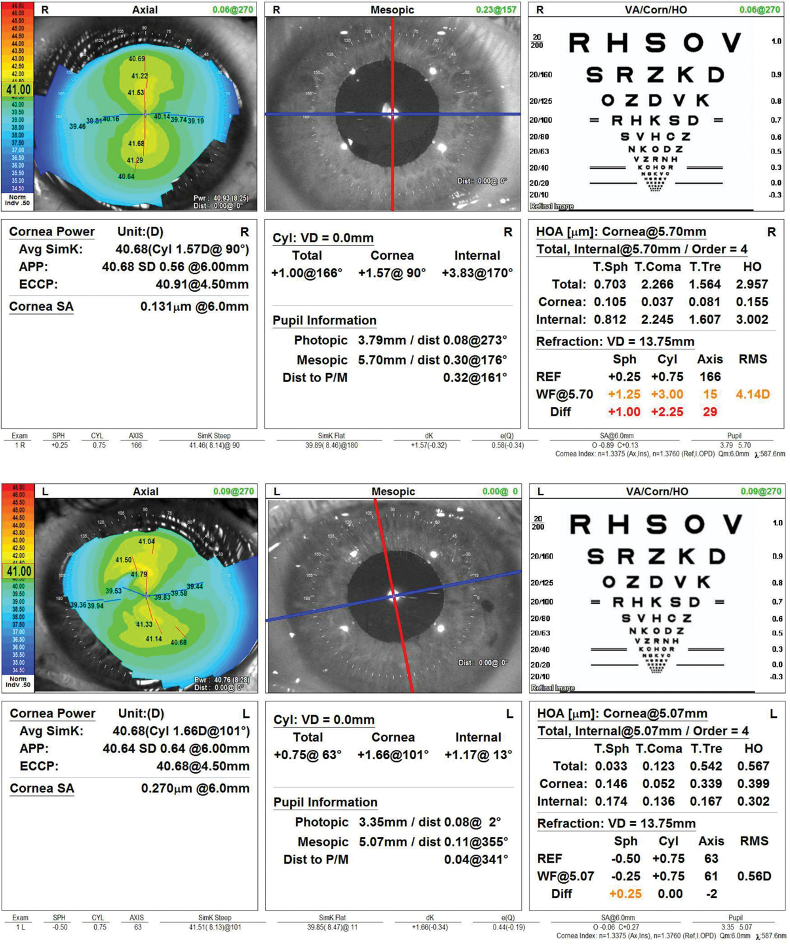
Figure 2. Computerized corneal topography of the right and left eyes.
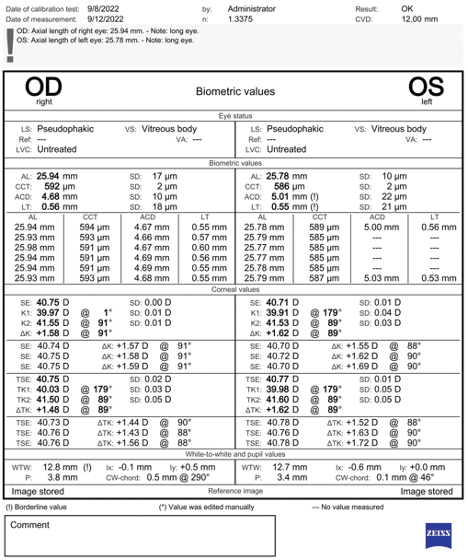
Figure 3. Biometry for both eyes.
How would you proceed? How would you manage the patient’s expectations?
— Case prepared by Ariel Chen, MD, and Brandon D. Ayres, MD

ZACHARY LANDIS, MD
A one-piece IOL with a haptic in the sulcus is never appropriate. There are already transillumination defects in the left eye, so time is of the essence. It would be important to hold an extensive discussion with the patient and set realistic expectations.
I would start by treating the left eye with a primary goal of positioning either a one-piece IOL completely in the capsular bag or a three-piece IOL in the sulcus, preferably with optic capture, to prevent further complications such as uveitis-glaucoma-hyphema syndrome and maximize the patient’s BCVA. Providing the best UCVA possible would be the secondary goal. The use of a toric trifocal IOL may not be possible. There is a risk of capsular compromise, and it may not be possible to execute the original plan depending on intraoperative findings.
Careful refraction and a comparison of pre- and postoperative biometry measurements would be prudent to determine the correct IOL power. Intraoperative aberrometry, if available, would be useful. If the current IOL is of the correct power and toricity and the patient refracts well with minimal spherical refractive error, then repositioning of the IOL can be planned, provided he understands an exchange for a monofocal lens may be necessary.
Intraoperatively, the anterior and posterior capsular leaflets would be carefully dissected for 360º with an OVD on a 26-gauge LASIK cannula. An attempt would be made to free the haptic in the bag to allow rotation of the IOL. If the IOL cannot be centered at the correct axis or if an exchange has already been planned, the IOL would be elevated into the anterior chamber, cut in half, and removed through a 3-mm incision. Care would be taken to maintain endothelial protection with a dispersive OVD.
There is a risk of violating the posterior capsule. Should this occur, an anterior vitrectomy would be performed, and a three-piece IOL would be placed in the sulcus. The capsulorhexis appears to be large, and optic capture may not be possible. An optic with a round anterior edge would therefore be preferable.

P. DEE G. STEPHENSON, MD, FACS, ABES, FSEE
The young patient has certainly been dealt a bad hand, but he has some options. Most important in this situation is to describe the options and possible secondary complications to him in detail and have him sign a consent form that lists the possibilities of an IOL exchange, vitrectomy, and visual complaints due to the chafing from the misplaced haptic.
Preparation would make any surgeon’s job easier in this situation. If possible, I would like to review the preoperative A-scan, the power of the lens implant, and preoperative keratometry readings to determine the intended postoperative refraction. If the IOL is correctly powered, simple redeposition of the sulcus-placed haptic may be sufficient. The capsular bag appears to be intact. One haptic is in the sulcus, and the other is in the bag. Gonioscopy and OCT imaging of the anterior angle would be performed to get a clearer picture of the situation.
I would be prepared to perform a pars plana vitrectomy if necessary. A main incision and two paracentesis incisions would be made to facilitate access. The capsular bag would be filled with an OVD. Next, an attempt would be made to dissect the anterior capsular flap away from the haptic in the sulcus with a blunt cannula and redeposit the haptic.
If the current IOL is the wrong power, the capsular bag would be filled with an OVD. Next, the lens would be disassembled with forceps (MicroSurgical Technology) and scissors and then removed, or it could be refolded and removed. If an IOL exchange is required, intraoperative aberrometry could be helpful.
It would be important to bear in mind that success with one eye does not guarantee success with the other.


WHAT WE DID: ARIEL CHEN, MD, AND BRANDON D. AYRES, MD
In this unusual case, both IOLs were malpositioned with one haptic in and one out of the capsular bag. The IOLs had also rotated off axis given the axis of corneal astigmatism. A lengthy discussion was had with the patient about repositioning the haptics in the capsular bag and rotating the IOL into the correct position. In situations like this one, the goal is to open the capsular bag and reposition the IOL in the bag. If the bag cannot be opened, the IOL must be exchanged for a three-piece lens to prevent the development of uveitis-glaucoma-hyphema syndrome. If the IOL must be exchanged, then it is preferable to fixate the IOL in the sulcus so that it cannot become dislocated. Before surgery, the risks and benefits of IOL repositioning and exchange and multiple surgical scenarios were discussed at length with the patient.
Surgical procedure. The right eye was addressed first. It was possible that an anterior vitrectomy and IOL exchange would be required, so a retrobulbar block with sedation was performed. The corneal axis was marked at the outset in case the toric IOL could be repositioned. Superior and inferior paracentesis incisions and a 3-mm temporal wound were created. The capsule was slowly dissected with an OVD to release the superior and inferior haptics.
The capsular bag could not be opened. It was therefore decided that the IOL would be exchanged. With forceps (MicroSurgical Technology) and intraocular scissors, the lens was cut into three pieces and removed through the main wound. Sulcus support was adequate, and the posterior capsule was intact. A posterior capsulorhexis was therefore created to allow optic capture of an IOL placed in the sulcus. An additional amount of an OVD was placed in the anterior chamber. A tear in the posterior capsule was created with a cystotome. A continuous curvilinear capsulorhexis of the posterior capsule was then performed with Utrata forceps (Figure 4). Care was taken to keep the anterior chamber deep and formed to prevent vitreous prolapse.
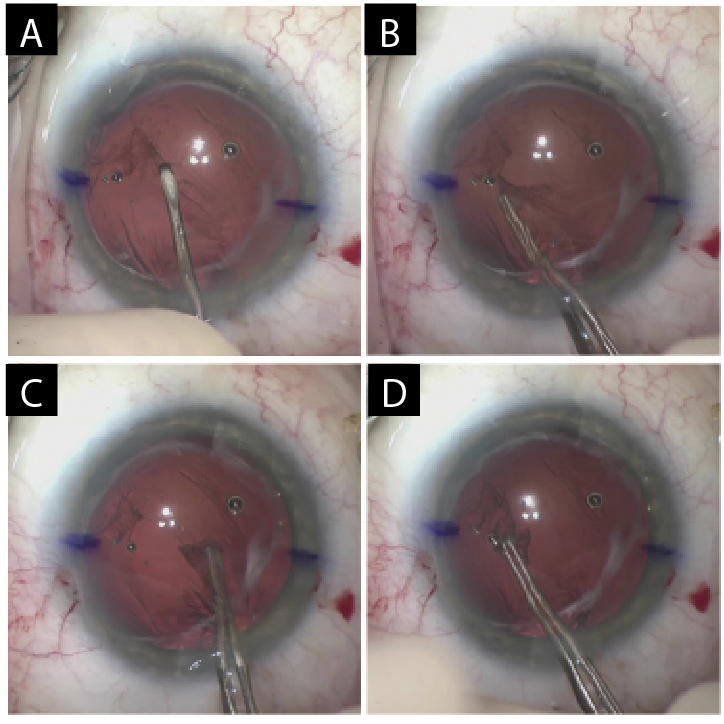
Figure 4. A cystotome is used to create a tear in the posterior capsule (A). Utrata forceps are used to create a continuous curvilinear capsulorhexis (B–D).
A CT Lucia (Carl Zeiss Meditec) was injected into the sulcus, and the optic was captured with the posterior capsulorhexis. Triamcinolone acetonide was injected into the anterior chamber. An anterior vitrectomy was performed. Acetylcholine (Miochol-E, Bausch + Lomb) was injected to constrict the pupil. The wounds were hydrated.
At the end of the case, the IOP was normotensive, the lens was well centered, the pupil was round, and the wounds were Seidel negative (Figure 5).
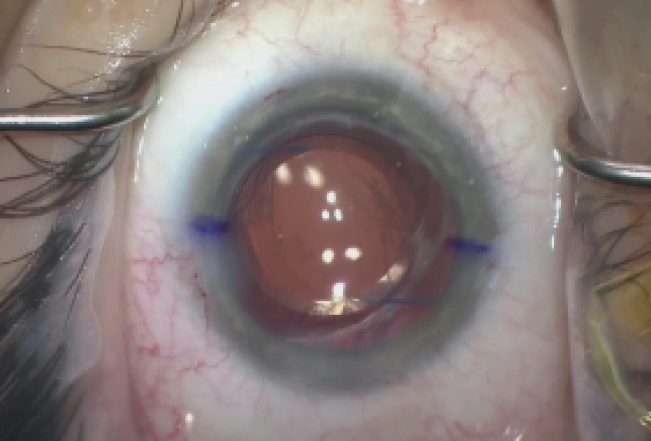
Figure 5. The immediate postoperative appearance of the left eye.
Postoperative course. One day after surgery, the patient’s UCVA was 20/60 OD. The IOP was 15 mm Hg with applanation tonometry. A slit-lamp examination found trace corneal stromal edema, a mild anterior chamber reaction, and a well-positioned IOL. A course of antibiotic and steroid drops dosed four times a day was initiated.
One week after surgery, the patient’s uncorrected distance visual acuity was 20/25 OD. He is happy with the visual results and plans to undergo surgery on the left eye after the right eye has healed fully.