Extreme myopia significantly challenges those affected by the condition in their daily activities. Spectacle lenses, being heavy and causing significant image minification, may not be compatible with sports and other physical activities. Contact lenses pose a continuous expense and risk of infection, and over time, some individuals become intolerant of them. For these reasons, many patients have described their experience with phakic IOLs as life changing. In this Fundamentals in Five, Patrick J. Pham, MD, and Robert T. Lin, MD , discuss crucial considerations for the EVO ICL (STAAR Surgical), a phakic IOL that was approved by the FDA in 2022.
Kavitha R. Sivaraman, MD


The EVO ICL is a posterior chamber phakic IOL indicated for the treatment of myopia. This removable implant is made from a biocompatible collagen polymer and does not cause the permanent corneal changes associated with typical laser refractive procedures.
The EVO ICL has demonstrated great efficacy and safety. In an FDA clinical trial of both spherical and toric implants, 90.5% of patients with a mean spherical equivalent of -7.62 D of myopia were within 0.50 D of target at 6 months and 87.6% achieved 20/20 UCVA or better.1 Moshirfar et al compared FDA outcomes with the ICL, SMILE, and topography-guided LASIK. The researchers concluded that all three procedures produced comparable UCVA at the 6- and 12-month intervals with a high degree of safety.2
1. IDENTIFYING SUITABLE CANDIDATES
The EVO ICL is indicated for the correction or reduction of stable moderate to severe myopia (spherical equivalent of -3.00 to -20.00 D) and up to 4.00 D of myopic astigmatism at the spectacle plane with the toric model. The lens has been approved by the FDA for patients between 21 and 45 years of age with an anterior chamber depth (ACD) of at least 3 mm and a suitable minimum endothelial cell count. The EVO also carries the European CE Mark for use in eyes with an ACD of at least 2.8 mm. Studies have demonstrated the implant’s safety.3,4
In our clinic, we consider an ICL for patients whose refraction approximates a -7.00 D spherical equivalent or greater and whose biometric parameters are appropriate. We also offer an ICL to individuals with lower prescriptions who have thin or suspicious corneas and to those who have dry eye disease. We are most confident implanting an ICL in eyes with an ACD of at least 2.8 mm, although we have safely implanted the lens in eyes with an ACD as shallow as 2.6 mm with appropriate preoperative counseling and postoperative monitoring.
2. ACCURATE SIZING
The sizing of an ICL is crucial because it determines the vault—the distance between the posterior surface of the implant and the anterior surface of the crystalline lens—and influences the implant’s rotational stability, which is paramount in the efficacy of astigmatic correction.
An ideal vault ranges from 250 to 750 µm. A vault of less than 250 µm increases the risk of anterior subcapsular cataract formation. Conversely, a vault greater than 750 µm could lead to pupillary block, angle crowding, and pigment dispersion that might lead to glaucoma and endothelial cell loss.
In our clinic, we take a conservative approach to vault sizing and favor the smaller implant if the eye falls between ICL sizes. Because the central 360-µm port of the EVO ICL preserves the physiologic circulation of aqueous humor, reports of visually significant anterior subcapsular cataracts following EVO ICL implantation are uncommon, suggesting an improved tolerance to lower vaults.5,6 When it comes to toric ICLs and eyes with an ACD of less than 2.8 mm, however, optimal sizing becomes critical because of the reduction of the anterior chamber angle (16º on average but can vary significantly). The risk of rotation appears to be related more to specific measurements such as sulcus-to-sulcus (STS) and angle-to-angle (ATA) rather than to vault. In our hands, we have observed about a 2% rate of rotation (unpublished data). Fortunately, this can be corrected easily with LASIK, limbal relaxing incisions, re-rotation, or ICL exchange.
3. ICL SIZING TECHNIQUES
Traditionally, external white-to-white (WTW) measurements have been used to select the ICL size. The STAAR Online Calculation and Ordering System uses a nomogram derived from WTW measurements obtained with an Orbscan tomographer (Bausch + Lomb). Studies have indicated a weak correlation between the WTW measurement and the actual STS diameter, however, which is the presumed location for lens placement.7 This discrepancy could lead to incorrect sizing and the related complications previously noted.
It stands to reason that a more precise measurement of the posterior chamber could enhance the accuracy of lens selection. Several research groups have sought to optimize ICL sizing by developing nomograms that draw upon measurements of the STS diameter.8,9 Recently, the inner diameter of the ciliary body and scotopic pupil size were also identified as significant predictors of postoperative vault and have been integrated into the process of ICL sizing.10
4. INCORPORATING PREOPERATIVE DIAGNOSTIC TESTING
Direct measurements of the anterior and posterior chambers play a pivotal role in identifying the optimal size for an ICL. Although ultrasound biomicroscopy can obtain these measurements, the handheld operation of the device often depends on user proficiency, which can lead to variability in results.
We favor an automated very high-frequency ophthalmic ultrasound device (Insight 100, ArcScan) for obtaining detailed biometric information with a repeatability of 0.12 mm when measuring behind the iris. In our experience, technicians can capture high-resolution images, yielding highly consistent results and thereby boosting our confidence in the measurements (Figure). These images enable us to discern critical parameters such as the STS distance, ciliary body inner diameter, and STS lens rise.

Figure. Preoperative biometric image captured with an automated very high-frequency ultrasound device. Calipers may be used to obtain many internal measurements, including the anterior chamber angle, ATA distance, STS distance, and ciliary body inner diameter.
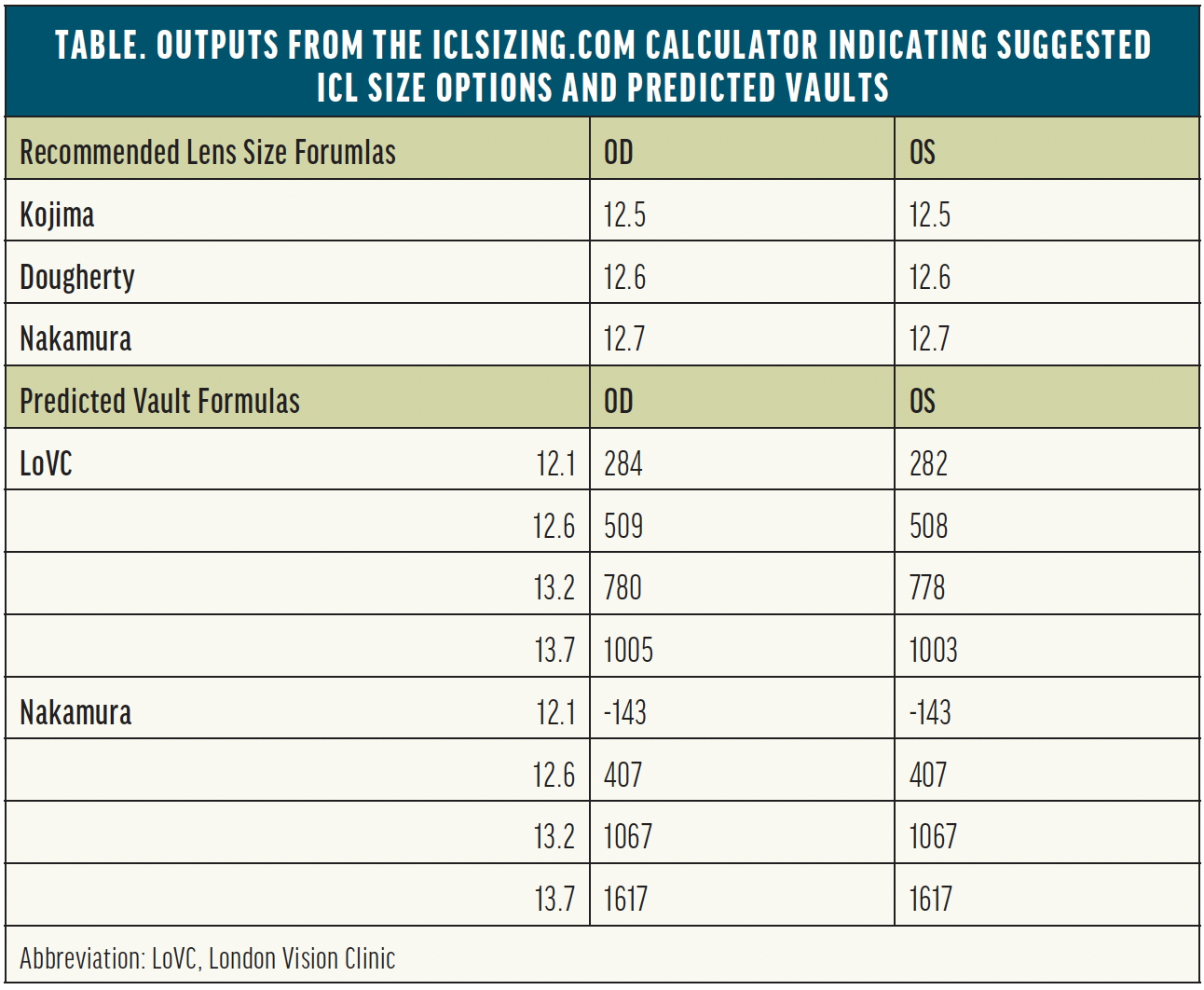
The parameters are fed into the iclsizing.com calculator developed by Dan Z. Reinstein, MD, MA(Cantab), FRCSC, DABO, FRCOphth, FEBO, and colleagues at the London Vision Clinic. The free tool not only suggests lens sizes based on the Kojima, Dougherty, and Nakamura nomograms, but it also predicts vaults for each potential implant size to guide surgical decision-making (Table).
5. POSTOPERATIVE CONSIDERATIONS
In addition to an evaluation of refractive outcomes, patients are routinely monitored for signs and symptoms that may suggest improper lens sizing. Systematic assessments of the anterior segment, including IOP measurements, lens vault, and a gonioscopic examination of the angle, are performed over time. A practical method by which to estimate vault at the slit lamp involves comparing the distance between the ICL and crystalline lens to the corneal thickness as viewed through a narrow slit beam. If an inappropriate vault is suspected, OCT or ultrasound imaging may be performed to measure it directly.
It is crucial to educate patients that, despite a successful refractive outcome, they remain at risk for ocular complications associated with their initial prescription. Individuals who had high myopia continue to be at increased risk of retinal detachment and early cataract development. Annual follow-up visits are therefore strongly recommended for these patients.
1. Packer M. Evaluation of the EVO/EVO+ sphere and toric Visian ICL: six month results from the United States Food and Drug Administration clinical trial. Clin Ophthalmol. 2022;16:1541-1553.
2. Moshirfar M, Somani AN, Motlagh MN, et al. Comparison of FDA-reported visual and refractive outcomes of the toric ICL lens, SMILE, and topography-guided LASIK for the correction of myopia and myopic astigmatism. J Refract Surg. 2019;35(11):699-706.
3. Silva R, Franqueira N, Faria-Correia F, Mendes J, Oliveira M, Monteiro T. Efficacy and safety after posterior chamber implantable collamer lens implantation according to preoperative anterior chamber depth: short-term comparative study. Eur J Ophthalmol. Published online October 9, 2022. doi:10.1177/11206721221131889
4. Niu L, Miao H, Han T, Ding L, Wang X, Zhou X. Visual outcomes of Visian ICL implantation for high myopia in patients with shallow anterior chamber depth. BMC Ophthalmol. 2019;19(1):121.
5. Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427-2438.
6. Wannapanich T, Kasetsuwan N, Reinprayoon U. Intraocular implantable collamer lens with a central hole implantation: safety, efficacy, and patient outcomes. Clin Ophthalmol. 2023;17:969-980.
7. Reinstein DZ, Archer TJ, Silverman RH, Rondeau MJ, Coleman DJ. Correlation of anterior chamber angle and ciliary sulcus diameters with white-to-white corneal diameter in high myopes using Artemis VHF digital ultrasound. J Refract Surg. 2009;25(2):185-194.
8. Kojima T, Yokoyama S, Ito M, et al. Optimization of an implantable collamer lens sizing method using high-frequency ultrasound biomicroscopy. Am J Ophthalmol. 2012;153(4):632-637, 637.e1.
9. Dougherty PJ, Rivera RP, Schneider D, Lane SS, Brown D, Vukich J. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13-18.
10. Reinstein DZ, Archer TJ, Vida RS, Piparia V, Potter JG. New sizing parameters and model for predicting postoperative vault for the implantable collamer lens posterior chamber phakic intraocular lens. J Refract Surg. 2022;38(5):272-279.