

Historically, patients with uncontrolled glaucoma who required cataract surgery either remained on their medications or underwent trabeculectomy or insertion of a glaucoma drainage device. Given the risk profile of traditional glaucoma surgery, intervention was typically reserved for severe or refractory disease and for patients who could not tolerate medical therapy. In the past decade, the rise of MIGS has given cataract surgeons and glaucoma specialists more options to address concomitant glaucoma at the time of cataract surgery.1
People use the term MIGS to describe a broad category of glaucoma treatments encompassing ab interno angle-based procedures that target the trabecular meshwork and Schlemm canal, ab interno procedures that target the supraciliary space, ab interno bleb-forming procedures, and endoscopic cyclophotocoagulation.2 This article discusses pre-, intra-, and postoperative considerations for patients undergoing combined cataract surgery and angle-based MIGS.
CONSIDERATIONS
History. In addition to the usual history-taking process during a cataract surgery evaluation, patients should be asked whether they are using aspirin and anticoagulation medications because both may increase bleeding-associated complications and affect the choice of procedure. Hyphema is the most common bleeding-associated complication after angle-based MIGS. Hyphema is usually self-limited and visually insignificant, but it can affect visual recovery and require anterior chamber washout.3 Patients with a history of allergic reaction to nitinol, nickel, or titanium should be counseled on the potential for allergy or hypersensitivity with trabecular meshwork bypass stents, and other alternatives should be discussed.
Gonioscopy. When evaluating a patient for cataract surgery combined with angle-based MIGS, gonioscopy is crucial to assess angle anatomy and make the correct diagnosis. The findings determine an individual’s candidacy and the expected efficacy of certain procedures.
Appositional closure suggests the primary angle-closure (PAC) spectrum, zonulopathy secondary to pseudoexfoliation (PXF), or trauma with a forward shift of the native lens.4 The presence of peripheral anterior synechiae (PAS) suggests PAC, uveitis, or trauma and calls into question a prior diagnosis of primary open-angle glaucoma (POAG). Eyes with extensive PAS may benefit from goniosynechialysis.5 Hyperopic eyes with a short axial length may be on the PAC spectrum.
Angle recession may signal a history of blunt trauma, which could increase the risk of zonular weakness and vitreous prolapse during cataract surgery.6 Myopic eyes with a long axial length sometimes have a physiologic wide ciliary body band, which may be difficult to distinguish from angle recession. Neovascularization in the angle should prompt a thorough retinal evaluation. It may be necessary to postpone cataract surgery until the underlying etiology of neovascularization has been treated.7
Functional testing. It is important to obtain a baseline visual field to determine the severity of vision loss.8 A printout of the baseline visual field facilitates discussion and can help glaucoma patients understand how much peripheral vision they have already lost and that the loss cannot be reversed by cataract surgery. Setting realistic expectations is important for building their trust in ongoing care. The extent of vision loss also determines the choice of MIGS procedure versus traditional glaucoma surgery.
Anesthesia. Most angle-based MIGS procedures are performed under topical anesthesia with monitored anesthesia care, similar to routine cataract surgery. Because their head must be turned to the side for angle surgery, patients must be able to follow instructions to look in primary gaze. Some may benefit from an additional sub-Tenon or retrobulbar block to minimize extraocular movement.9
CHOICE OF SURGERY BY GLAUCOMA SEVERITY AND TYPE
Mild to moderate glaucoma. For someone with mild to moderate disease that is well controlled on medical therapy, it may be reasonable to offer cataract surgery alone or in combination with angle-based MIGS. A stronger recommendation for MIGS can be made to patients who are unable to tolerate, adhere to, or afford prescribed medical therapy. Many cataract surgeons have the experience and skills required to address glaucoma in this patient population.
Stents that bypass the trabecular meshwork such as the iStent inject (Glaukos; Figure A) and Hydrus Microstent (Alcon) are indicated for use in patients with mild to moderate POAG at the time of cataract surgery. Two-year results from the pivotal iStent Inject trial and 5-year results from the HORIZON trial demonstrated that cataract surgery combined with the placement of two iStent injects or one Hydrus Microstent, respectively, resulted in a higher proportion of eyes with an unmedicated IOP of 18 mm Hg or less and a higher proportion of eyes that achieved an IOP reduction of at least 20% without medications compared to cataract surgery alone.10,11
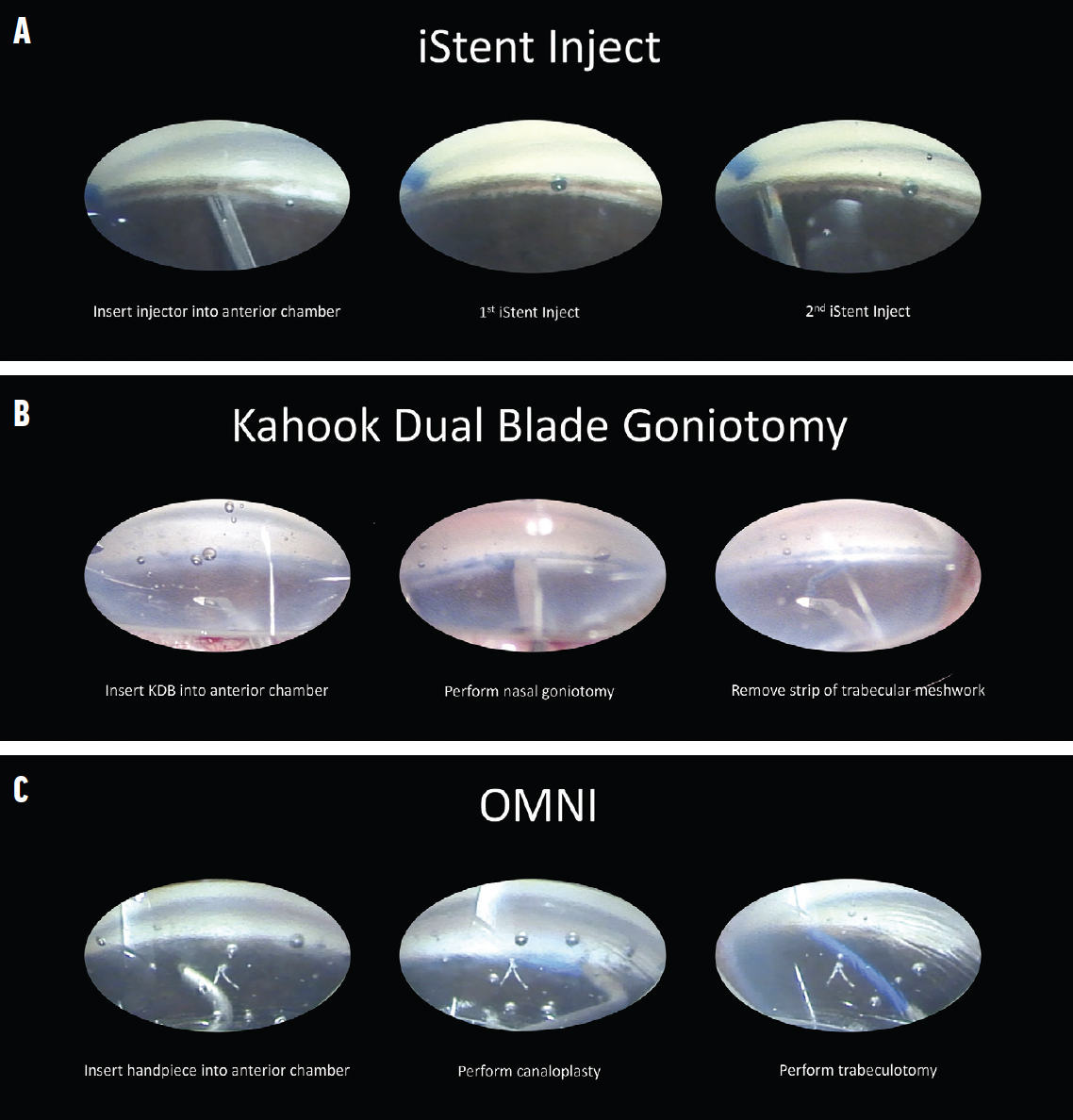
Figure. Overview of surgical steps with the iStent inject (A), Kahook Dual Blade (B), and Omni Surgical System (C).
Other angle-based MIGS procedures may be performed at the time of cataract surgery to address a range of glaucoma etiologies and stages. The procedures include goniotomy and ab interno canaloplasty with instruments such as the Kahook Dual Blade (Figure B) and Streamline (both from New World Medical), TrabEx (Microsurgical Technology), Omni Surgical System (Figure C) and Sion (both from Sight Sciences), and iTrack (Nova Eye Medical). A literature review found goniotomy performed with the Kahook Dual Blade and combined with cataract surgery to be effective at lowering IOP and the medication burden across a spectrum of glaucoma severity and types.12
Intraoperatively, patients with mild to moderate glaucoma likely have sufficient optic nerve reserve to tolerate a transient IOP elevation. Thorough OVD removal, however, should be performed at the conclusion of combined surgery, as is typically done during routine cataract surgery, to avoid a postoperative IOP spike.
If standalone cataract surgery is performed, it may be reasonable for patients to resume baseline glaucoma medications on the night of surgery or starting the next day. If a combined procedure is performed, it may be reasonable to stop baseline glaucoma medications and assess the new, untreated IOP before determining if glaucoma therapy should be resumed. Patients who experience an IOP spike on postoperative day 1 may need an anterior chamber tap or OVD release through the paracentesis site.13 Some surgeons prescribe an oral carbonic anhydrase inhibitor on the night of surgery to reduce the risk of a postoperative IOP spike.14 It is important to bear in mind, however, that angle-based procedures may be associated with reflux bleeding if the IOP drops below the episcleral venous pressure and that, at times, the benefit of a transient IOP elevation outweighs the risk of a reflux hyphema.3
It can be advantageous for cataract surgeons to become familiar and gain experience with one or more MIGS procedures for the treatment of mild to moderate glaucoma and refer patients who fall outside this scope to a glaucoma specialist.
Severe or refractory glaucoma. For someone with severe glaucoma that is well controlled and who is tolerating current medical therapy, it is reasonable to offer cataract surgery alone or in combination with MIGS or to refer them to a glaucoma surgeon for cataract surgery combined with traditional glaucoma surgery. Some patients with dense or high-risk cataracts may opt for cataract surgery alone and defer glaucoma surgery. Others may prefer combined surgery to reduce their medication burden.
The iStent infinite (Glaukos) is indicated for the treatment of severe glaucoma. Goniotomy and canaloplasty are additional options.15 Insurance coverage, however, may present a challenge.
Patients with severe glaucoma have less optic nerve reserve to tolerate even a transient IOP spike. Informed consent should include the risk of snuff-out, a permanent and severe loss of vision.16 It may be reasonable to prescribe a systemic carbonic anhydrase inhibitor before, during, or after surgery to prevent an IOP spike.
Cataract surgeons generally can perform cataract surgery combined with MIGS on a patient with well-controlled, severe glaucoma. Patients, however, should be counseled about the possibility of a future referral to a glaucoma specialist if the IOP reduction is inadequate.
If advanced glaucoma is uncontrolled and the patient might benefit from traditional glaucoma surgery at the time of cataract surgery, a referral to a glaucoma surgeon is warranted. Trabeculectomy and tube shunt surgery are options depending on the patient’s clinical characteristics and the glaucoma specialist’s experience and practice patterns.17,18
A Xen Gel Stent (Allergan/AbbVie) may be an option for patients with severe or refractory glaucoma.18 Some cataract surgeons perform a moderate volume of the procedure. It is important to note that Xen implantation with bleb formation may have a different risk profile than other angle-based MIGS procedures in terms of failure and the need for additional intervention. A referral to a glaucoma specialist may therefore be in the patient’s best interest if a Xen procedure is being considered.
When performing cataract surgery on an eye with a history of trabeculectomy, a subconjunctival injection of 5-fluorouracil may maintain the viability of a functioning bleb.19 Anterior chamber stability may be poor owing to fluid egress through the trabeculectomy ostomy, requiring the injection of high-density OVDs.
When performing cataract surgery on an eye with a tube shunt, modifications and adjustments may be required. A technique has been described to occlude the tube temporarily with an iris hook to prevent the flow of lens debris and OVD.20 If the tube is too long and interfering with various steps of cataract surgery, iris hooks may be used to retract the tissue near the location of the tube, and the tube may be tucked into this space to keep it out of the way. Alternatively, a long tube may be trimmed. If the position in the anterior chamber is suboptimal, the tube may also be repositioned into the ciliary sulcus.21 If extensive revision of a prior trabeculectomy or tube is required at the time of cataract surgery, patients may be referred to a glaucoma specialist.
Other types of glaucoma. Eyes with suspected or confirmed PAC or PAC glaucoma may benefit from early cataract surgery for a visually significant cataract or clear lens extraction if they meet the criteria outlined in the EAGLE trial (ie, PAC with an IOP > 30 mm Hg or PAC glaucoma).22
Eyes with a very short axial length may benefit from intravenous mannitol in the preoperative area to decompress the vitreous and minimize posterior pressure. Heavy OVDs such as Healon GV and Healon 5 (both from Johnson & Johnson Vision) can help maintain anterior chamber depth.
Patients with plateau iris syndrome or extensive PAS may not be suitable candidates for angle surgery and may benefit from endoscopic cyclophotocoagulation at the time of cataract surgery.23
Eyes with PXF syndrome or PXF glaucoma are at increased risk of miosis and zonulopathy; the surgeon should be prepared to place pupil expansion devices and capsular tension rings and segments. Eyes with uveitic glaucoma should be quiet before cataract surgery is performed, and steroids may be required before, during, or after surgery to prevent rebound inflammation.24 Eyes with neovascular glaucoma require pretreatment with antivascular endothelial growth factor injections and/or panretinal photocoagulation to minimize active anterior segment neovascularization before cataract surgery.
For patients with other coexisting eye diseases, multidisciplinary collaboration with other specialists is critical to optimizing long-term success and minimizing surgical complications.25
1. Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104.
2. Fellman RL, Mattox C, Singh K, et al. American Glaucoma Society position paper: microinvasive glaucoma surgery. Ophthalmol Glaucoma. 2020;3(1):1-6.
3. Pratte EL, Ramachandran M, Landreneau JR, An JA. Risk factors for hyphema following Kahook Dual Blade goniotomy combined with phacoemulsification. J Glaucoma. 2023;32(3):165-170.
4. Gedde SJ, Chen PP, Muir KW, et al; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary angle-closure disease preferred practice pattern. Ophthalmology. 2021;128(1):P30-P70.
5. Ahmed IIK, Durr GM. Goniosynechialysis … to release or not to release? That is not the question. Ophthalmol Glaucoma. 2019;2(5):277-279.
6. Tumbocon JAJ, Latina MA. Angle recession glaucoma. Int Ophthalmol Clin. 2002;42(3):69-78.
7. Aboobakar IF, Lin MM. Clinical diagnosis of neovascular glaucoma in the ophthalmology office. In: Qiu M, ed. Neovascular Glaucoma. Essentials in Ophthalmology. Springer; 2022:23-29.
8. Prum Jr BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2016;123(1):P41-P111.
9. Chua MJ, Lersch F, Chua AWY, Kumar CM, Eke T. Sub-Tenon’s anaesthesia for modern eye surgery—clinicians’ perspective, 30 years after re-introduction. Eye (Lond). 2021;35(5):1295-1304.
10. Ahmed IIK, De Francesco T, Rhee D, et al; HORIZON Investigators. Long-term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129(7):742-751.
11. Samuelson TW, Sarkisian SR, Lubeck DM, et al; iStent inject Study Group. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811-821.
12. Dorairaj S, Radcliffe NM, Grover DS, Brubaker JW, Williamson BK. A review of excisional goniotomy performed with the Kahook Dual Blade for glaucoma management. J Curr Glaucoma Pract. 2022;16(1):59-64.
13. Zebardast N, Zheng C, Jampel HD. Effect of a Schlemm’s canal microstent on early postoperative intraocular pressure after cataract surgery: an analysis of the HORIZON randomized controlled trial. Ophthalmology. 2020;127(10):1303-1310.
14. Hayashi K, Yoshida M, Sato T, Manabe SI, Yoshimura K. Intraocular pressure elevation after cataract surgery and its prevention by oral acetazolamide in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg. 2018;44(2):175-181.
15. Sarkisian SR Jr, Grover DS, Gallardo MJ, et al; iStent infinite Study Group. Effectiveness and safety of iStent Infinite trabecular micro-bypass for uncontrolled glaucoma. J Glaucoma. 2023;32(1):9-18.
16. Mohammadzadeh V, Galian K, Martinyan J, Nouri-Mahdavi K. Vision loss after glaucoma surgery: progressive macular thinning as a sign of snuff-out phenomenon. J Glaucoma. 2019;28(6):e99-e102.
17. Gedde SJ, Feuer WJ, Chen PP, Heuer DK, Singh K, Wright MM; Tube Versus Trabeculectomy and Primary Tube Versus Trabeculectomy Study Groups. Comparing treatment outcomes from the Tube Versus Trabeculectomy and Primary Tube Versus Trabeculectomy studies. Ophthalmology. 2021;128(2):324-326.
18. Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN Gel Stent: a comprehensive review on its use as a treatment option for refractory glaucoma. Clin Ophthalmol. 2020;14:1805-1832.
19. Ng WS, Jayaram H. Adjunctive modulation of wound healing during cataract surgery to promote survival of a previous trabeculectomy. Cochrane Database Syst Rev. 2021;8(8):CD013664.
20. Välimäki J. Cataract surgery in patients with glaucoma drainage implants: the hooked tube technique. Int Ophthalmol. 2019;39(3):605-610.
21. Weinreb S, Cardakli N, Jefferys J, Quigley H. Long-term functional outcomes of glaucoma tube shunt revision surgery. Ophthalmol Glaucoma. 2019;2(6):383-391.
22. Azuara-Blanco A, Burr J, Ramsay C, et al; EAGLE study group. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389-1397.
23. Hollander DA, Pennesi ME, Alvarado JA. Management of plateau iris syndrome with cataract extraction and endoscopic cyclophotocoagulation. Exp Eye Res. 2017;158:190-194.
24. Chen RI, Purgert R, Eisengart J. Gonioscopy-assisted transluminal trabeculotomy and goniotomy, with or without concomitant cataract extraction, in steroid-induced and uveitic glaucoma: 24-month outcomes. J Glaucoma. 2023;32(6):501-510.
25. Qiu M, Shukla AG, Sun CQ. Improving outcomes in neovascular glaucoma. Ophthalmol Glaucoma. 2022;5(2):125-127.




