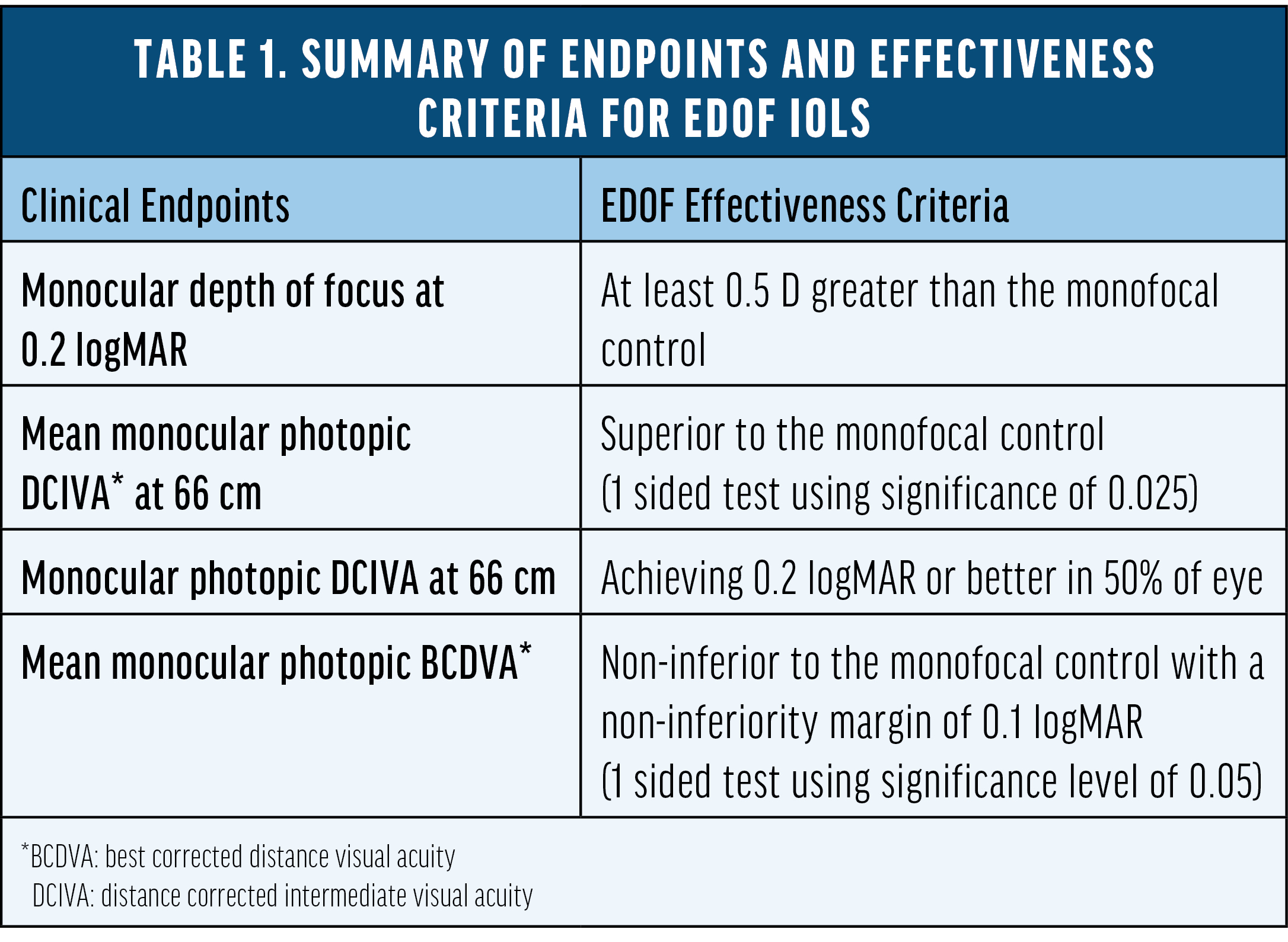
Introduction
Despite the significant advancement of presbyopia-correcting IOL technology, monofocal IOLs—which are designed to restore distance vision at a lower cost and are covered by medical insurance—remain the most implanted type of IOL worldwide. Recently, certain monofocal IOLs with modified aspheric optical profiles, reportedly designed to slightly extend the depth of focus, have been introduced to the market.1 It is important to understand the range of vision these monofocal lenses demonstrate versus other traditional monofocal IOLs, particularly given the associated additional cost.
Traditional Aspheric Monofocal IOLs
The optical design of monofocal IOLs has long been focused on providing high-quality distance vision. They were originally developed with spherical surfaces. Unlike the young human crystalline lens, which provides negative spherical aberration (SA)2 and can neutralize positive corneal SA,3 spherical IOLs introduce additional positive SA.4 Increased ocular SA reduces the clarity of an image as the aberrations cause light to deviate from the point of focus on the retina.4 The desire to provide sharply focused distance vision prompted the development of aspheric monofocal IOLs, which feature a gradual curvature reduction from center to the periphery, resulting in concomitant change in optical power from center to the peripheral portions of the optical zone.4 Today, we have a collection of aspheric lenses that are designed to neutralize all (e.g., TECNIS ZCB00 [Johnson & Johnson Vision], -0.27 μm SA), partial (e.g., AcrySof IQ SN60WF, Clareon SY60WF [both Alcon], -0.2 μm SA), or none (e.g., Akreos AO, enVista MX60E [both Bausch + Lomb], 0 μm SA) of the visual system’s naturally occurring corneal SA (+0.28 ± 0.09 μm for a 6-mm pupil).3 Different aspheric monofocal IOLs may be selected based on a patient’s corneal higher-order aberration profile to achieve higher-quality distance vision.
It should be noted that residual SA has the potential benefit of providing some depth of focus or tolerance to residual refractive error when it is not high enough to degrade retinal image quality. Patients with traditional monofocal IOLs, especially spherical or aspheric IOLs that do not fully neutralize corneal SA, have been reported to provide some depths of focus.5-8
Modified Monofocal IOLs
Recently, a group of modified monofocal IOLs has emerged with optical profiles designed to extend the depth of focus slightly. However, Fernandez et al9 showed that none of these lenses are clinically proven to meet the extended depth-of-focus (EDOF) IOL standard as defined by the American National Standard Z80.35-2018 (Table 1).10 These lenses are approved as monofocal IOLs and marketed to slightly extend the depth of focus. TECNIS Eyhance (Johnson & Johnson Vision), for example, is an FDA-approved monofocal IOL with a modified aspheric anterior surface which facilitates a steady increase in lens power within the central 1-mm diameter of the IOL optic. It was introduced as a level A modification of the TECNIS ZCB00 monofocal IOL, and did not require additional clinical study to verify that the modified optical design provided any additional benefit. Different studies have compared Eyhance to its parent lens, the TECNIS ZCB00, and showed various levels in intermediate visual acuity with mean value ranging from 0.09 to 0.2 logMAR.11-13 However, limited data is available comparing the range of vision between Eyhance and other traditional aspheric monofocal IOLs.
As mentioned above, having a range of vision with monofocal IOLs is not a novel concept nor a newly studied phenomenon. Rocha et al5,6 showed residual SA with spherical IOLs and aspherical neutral IOLs could improve depth of focus. Bilateral implantation of AcrySof IQ monofocal IOLs has also been shown to provide intermediate visual acuity of 0.2 logMAR.7,8 The Clareon monofocal IOL is a recent innovation from Alcon with an advanced biomaterial to provide excellent optical clarity.14 It shares a similar optical design as the AcrySof IQ monofocal IOL with -0.2 μm asphericity to partially compensate corneal SA, suggesting it may provide similar range of vision as the AcrySof IQ monofocal IOL. Blehm et al reported distance and intermediate visual acuity (mean value of 0.16 and 0.23 logMAR at 80 cm and 66 cm, respectively) from a prospective study with the Clareon monofocal IOL.15
To better understand the range of vision of the Eyhance monofocal IOL versus an aspheric monofocal IOL other than TECNIS ZCB00, we conducted a large, non-interventional, single-center, multisurgeon, head-to-head study comparing visual acuity outcomes in patients bilaterally implanted with either Clareon or Eyhance monofocal IOLs.16 Non-inferiority of the Clareon monofocal IOL relative to the Eyhance monofocal IOL was demonstrated in both BCDVA and DCIVA measured at 66 cm (Table 2) in an apples-to-apples comparison with both groups corrected to plano. A 2.5-ETDRS letter difference in DCIVA was observed between the two IOLs, which is well within the range of non-inferiority (<0.1 logMAR) and not considered to be a clinically meaningful difference. The binocular distance corrected defocus curve measurements were extremely similar from -3.00 D to +1.00 D defocus level (P > 0.05), suggesting comparable range of vision achieved by the two monofocal IOLs (Figure 1).
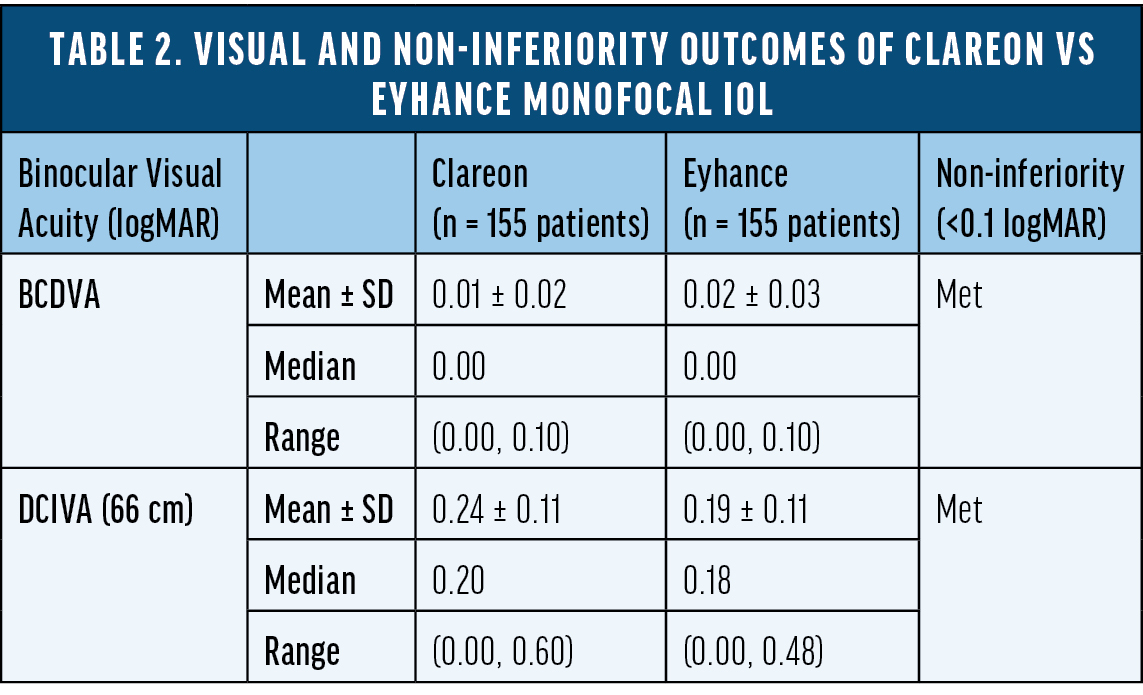
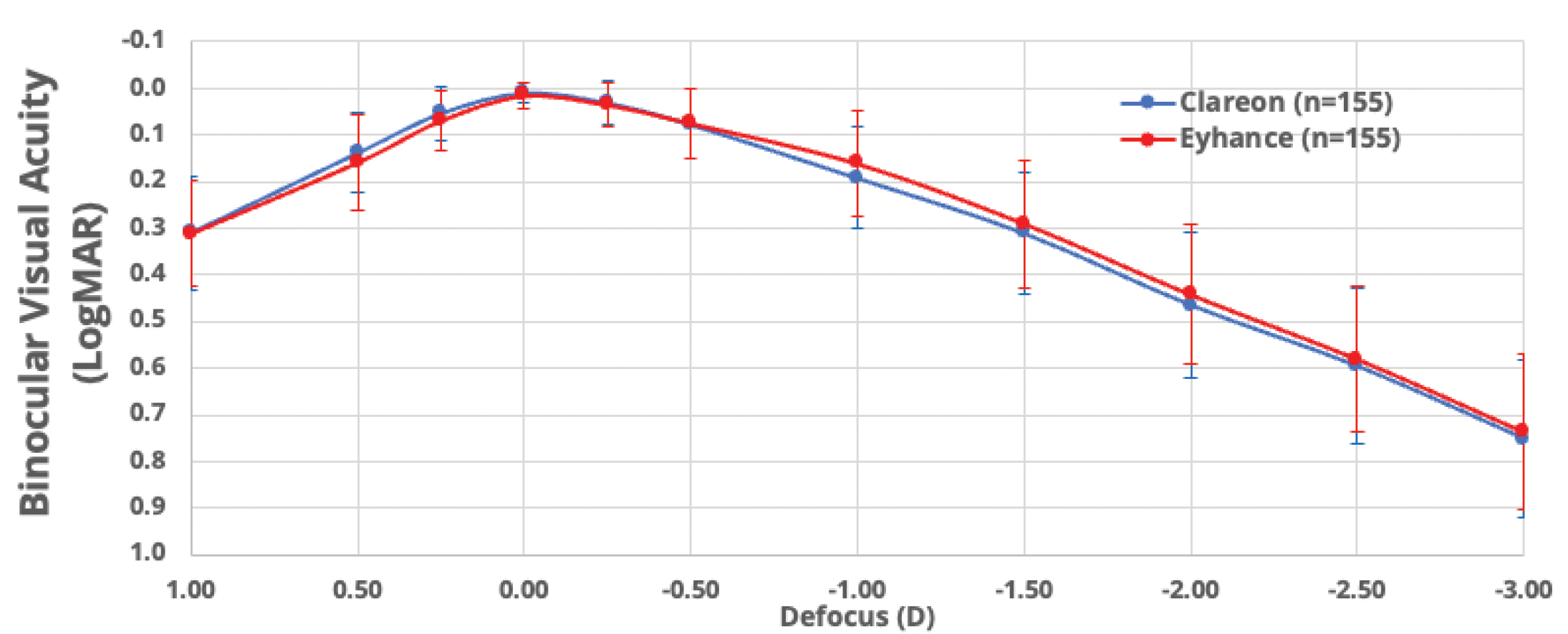
Figure 1. Binocular distance-corrected defocus curve of Clareon vs Eyhance monofocal IOL.
Additionally, it's crucial to underscore the importance of evaluating distance-corrected visual acuities at the relevant point of focus—distance, intermediate, or near—when comparing the visual performance of two IOLs. This approach allows us to rule out the influence of residual refractive error, ensuring a fair comparison of the visual benefits each IOL provides.
Summary
Monofocal IOLs remain an important option for cataract patients, typically providing excellent distance VA. The Eyhance monofocal IOL has been demonstrated to have slightly better intermediate VA compared to its parent monofocal IOL. However, this observation cannot be generalized to all monofocal IOLs. Our data conclude that the Clareon monofocal IOL has a comparable range of vision compared to the Eyhance monofocal IOL. The Clareon monofocal IOL may be an equally suitable choice with a relatively lower cost for surgeons who want to provide patients with similar range of vision after cataract surgery. For patients who desire excellent vision from distance to intermediate and functional near vision with a low incidence of visual disturbance, non-diffractive EDOF IOLs should be considered. They are known to consistently deliver extended range of vision without the dependency on targeting strategies like mini-mono or monovision.7,8
The views and opinions expressed in this content may not necessarily represent those of Bryn Mawr Communications or Cataract & Refractive Surgery Today.
Important Product Information - Clareon® Family of IOLs
CAUTION: Restricted by law to sale by or on the order of a physician.
DESCRIPTION: The Clareon® Family of Lenses are artificial lenses implanted in the eye of adult patients following cataract surgery. The Clareon® Aspheric Hydrophobic Acrylic IOLs are designed to allow for clear distance vision. However, you will likely still need glasses for reading and for distance vision particularly if you already have astigmatism. The Clareon® PanOptix® Trifocal Hydrophobic IOL is a type of multifocal lens (sometimes called “presbyopia-correcting IOL”) designed to allow for clear distance, intermediate, and near vision with the potential to be more independent of the need to use glasses for daily tasks. The Clareon® Vivity™ Extended Vision Hydrophobic Posterior Chamber IOL provides clear distance vision, and better intermediate and some near vision compared to a monofocal IOL. The Clareon® Aspheric Toric, Clareon® PanOptix® Toric, and Clareon® Vivity™ Toric IOLs are also designed to correct pre-existing corneal astigmatism, which is the inability of the eye to focus clearly at any distance because of difference curvatures on the cornea, and provide distance vision.
WARNINGS / PRECAUTIONS: You may experience and need to contact your eye doctor immediately if you have any of the following symptoms after cataract surgery: itching, redness, watering of your eye, sensitivity to light. The safety and effectiveness of these IOLs have not been established in patients with eye conditions, such as an increase in eye pressure (glaucoma) or complications of diabetes in the eye (diabetic retinopathy). As with any surgical procedure, there are risks involved. These risks may include but are not limited to infection, damage to the lining of the cornea, the retinal layer which lines the inside back wall of your eye may become separated from the tissue next to it (retinal detachment), inflammation or swelling inside or outside the eye, damage to the iris (the colored diaphragm around the pupil), or an increase in eye pressure that cannot be controlled by medicine and secondary surgical procedure. There is a possibility that the IOL may be placed incorrectly or could move within the eye. This may result in less improvement or a reduction in vision, or it may cause visual symptoms. The Clareon® Aspheric Toric, Clareon® PanOptix® Toric, and Clareon® Vivity™ Toric IOLs correct astigmatism only when placed in the correct position in the eye. There is a possibility that these Toric IOLs could be placed incorrectly or could move within the eye. This may result in less improvement or a reduction in vision because your astigmatism has not been fully corrected, or it may cause visual symptoms. With the Clareon® PanOptix® and Clareon® Vivity™ IOLs, there may be a loss of sharpness of your vision that may become worse in dim light or in foggy conditions. There is also a possibility that you may have some visual effects such as rings or circles (halos) around lights at night. You may also have trouble seeing street signs due to bright lights or glare from oncoming headlights.
ATTENTION: As with any surgical procedure, there are risks involved. Prior to surgery, ask your eye doctor to provide you with the Patient Information Brochure for the lens to be implanted. This Brochure which will inform you of the risks and benefits associated with the IOL. Discuss any questions about possible risks and benefits associated with your eye doctor.
1. TECNIS Eyhance DFU.
2. Smith G, Cox MJ, Calver R, Garner LF. The spherical aberration of the crystalline lens of the human eye. Vision Res. 2001;41(2):235-243.
3. Wang L, Dai E, Koch DD, Nathoo A. Optical aberrations of the human anterior cornea. J Cataract Refract Surg. 2003;29(8):1514-1521.
4. Holladay JT, Piers PA, Koranyi G, van der Mooren M, Norrby NE. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J Refract Surg. 2002;18(6):683-691.
5. Rocha KM, Soriano ES, Chamon W, Chalita MR, Nosé W. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: a prospective randomized study. Ophthalmology. 2007;114(11):2050-2054.
6. Rocha KM, Gouvea L, Waring GO 4th, Haddad J. Static and dynamic factors associated with extended depth of focus in monofocal Intraocular Lenses. Am J Ophthalmol. 2020;216:271-282.
7. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a U.S. registration study of a new EDOF intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48(11):1297-1304.
8. Bala C, Poyales F, Guarro M, et al. Multicountry clinical outcomes of a new nondiffractive presbyopia-correcting IOL. J Cataract Refract Surg. 2022;48(2):136-143.
9. Fernández J, Rocha-de-Lossada C, Zamorano-Martín F, Rodríguez-Calvo-de-Mora M, Rodríguez-Vallejo M. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: a scoping review. BMC Ophthalmol. 2023;23(1):101.
10. American National Standard for Ophthalmics. ANSI Z80.35-2018: extended depth of focus intraocular lenses. 2018.
11. Auffarth GU, Gerl M, Tsai L, Janakiraman DP, Jackson B, Alarcon A, Dick HB; Quantum Study Group. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg. 2021;47(2):184-191.
12. Mencucci R, Cennamo M, Venturi D, Vignapiano R, Favuzza E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg. 2020;46(3):378-387.
13. Unsal U, Sabur H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int Ophthalmol. 2021;41(1):273-282.
14. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45(10):1490-1497.
15. Blehm C, Hall B. Evaluation of visual outcomes and 3-month refractive stability of a new hydrophobic acrylic intraocular lens. Clin Ophthalmol. 2023;17:1859-1864.
16. Micheletti JM. Head-to-head comparison of two monofocal IOL. Presented at the annual meeting of the ASCRS. San Diego, CA. May 2023.
© 2024 Alcon Inc. 3/24 US-CLM-2400001

