
Premium IOLs are increasingly being used in cataract surgery and refractive lens exchange (RLE) with the intention of correcting presbyopia and refractive error. Patients undergoing RLE with a multifocal IOL have high expectations and demands for greater spectacle independence and improved quality of life after surgery.
A multitude of factors can influence the performance of premium IOLs. One of the commonly cited reasons for dissatisfaction with multifocal lenses is residual refractive astigmatism.1,2 It is estimated that more than 30% of cataract surgery candidates have preexisting corneal astigmatism of 1.00 D or more.3 Concurrent management of astigmatism during cataract or RLE surgery is, therefore, critical.4
Some residual astigmatism following intraocular surgery is not uncommon, and there is a need for greater understanding of its effect on patients’ visual function and satisfaction. This is particularly important in pseudophakic patients with multifocal IOLs, for whom the performance of the lens can be compromised due to the presence of a refractive error.
In an effort to understand the effect of residual astigmatism on patient satisfaction with premium IOL implantation, we took advantage of the large dataset available from the Optical Express (United Kingdom) patient database. This article describes how these data allowed us to determine the importance of controlling residual astigmatism to achieve good outcomes and happy patients.
LARGE SAMPLE
A large sample of patients bilaterally implanted with multifocal IOLs and available for the 3-month postoperative visit (17,386 eyes of 8,693 patients) was extracted from the Optical Express dataset to study the relationship between residual astigmatism and visual function and satisfaction (all patient identifiers were redacted; unpublished data). These patients were bilaterally implanted with multifocal IOLs from different manufacturers, including diffractive IOLs with various near add strengths and extended depth of focus IOLs. The mean age of the study group was 58.1 ±7.2 years. In this sample, 68.6% of eyes had preoperative corneal astigmatism of 0.50 D or more, and 24% had 1.00 D more. Similarly, 65.7% and 21.2% of eyes, respectively, had preoperative refractive astigmatism of 0.50 D or more and 1.00 D or more.
Preexisting corneal astigmatism was managed at the time of surgery according to its magnitude. Low amounts of corneal astigmatism (< 0.75 D) were generally managed by creating a clear corneal incision on the steepest meridian. In eyes with corneal astigmatism of 0.75 to 1.50 D, opposite clear corneal incisions, limbal relaxing incisions, or laser-assisted astigmatic keratectomy on the steep axis was typically performed. A toric IOL was used in patients with corneal astigmatism of 1.50 D or more.
CLEAR ASSOCIATION
At 3 months postoperative, patients achieved good visual acuity: 86.1% of patients had 20/20 or better binocular uncorrected distance visual acuity (UDVA). Mean refractive cylinder was reduced from -0.55 ±0.45 D preoperatively to -0.43 ±0.39 D at 3 months (P < .01). Among all eyes, 73% had residual postoperative refractive cylinder of 0.50 D or less, 94.8% had 1.00 D or less, and 99.8% had 2.00 D or less.
Figure 1 depicts the relationship between monocular UDVA and residual refractive cylinder at 3 months at the 20/20 and 20/16 levels. There is an obvious association, with reduction of visual acuity with increasing levels of residual astigmatism. This is quite apparent even for low amounts of residual astigmatism. For example, a significant drop in monocular UDVA was observed between eyes with no refractive astigmatism (80.5% of which achieved monocular UDVA 20/20 or better) and those with 0.50 D of residual astigmatism (66.2% of which achieved 20/20 or better).
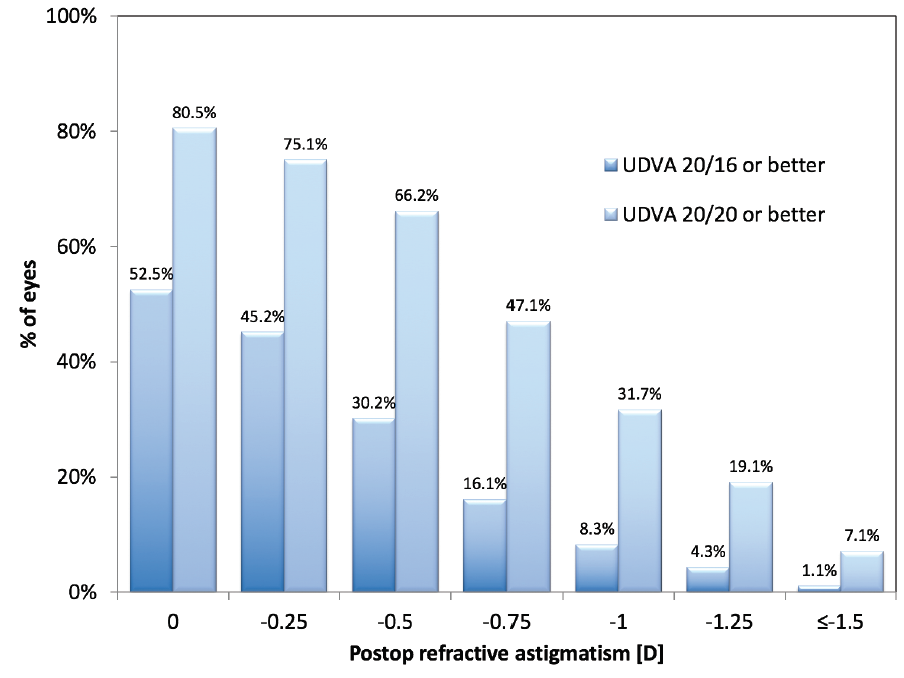
Figure 1. Monocular UDVA versus residual refractive astigmatism at 3 months postoperative.
The presence of residual astigmatism in one or both eyes of a patient also affected binocular UDVA. Stratification of binocular visual acuity was performed based on the residual cylinder in the dominant eye and in the worse eye (the eye of the patient with the higher magnitude of residual astigmatism). A gradual decrease in binocular UDVA was observed with increasing astigmatism (Figure 2). Generally, binocular UDVA of 20/20 or better was more affected by astigmatism in the dominant eye, where the UDVA values were lower compared with stratification by worse eye. Only half of the patients with residual cylinder of 1.50 D or more in one of their two eyes achieved binocular UDVA of 20/20 or better.
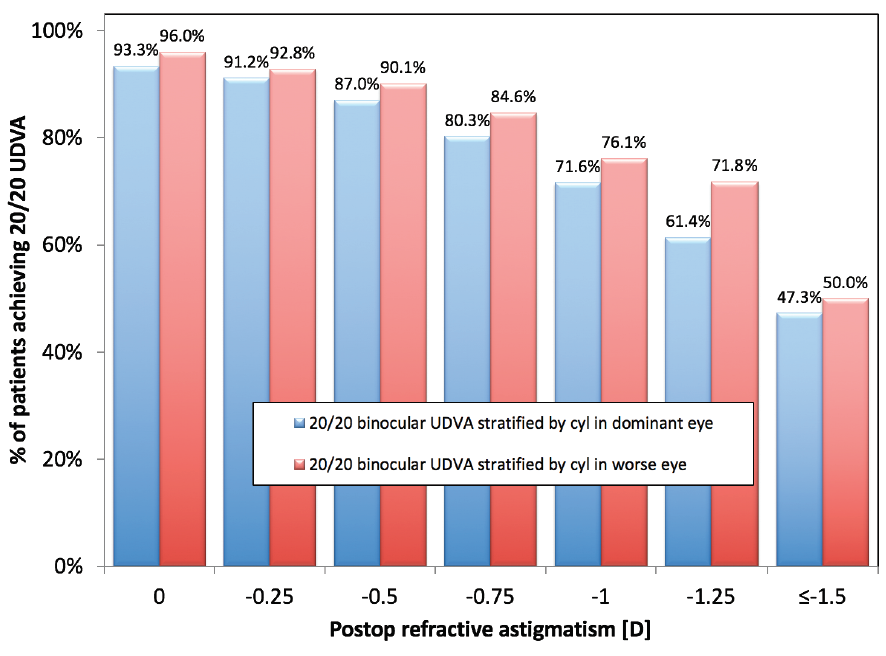
Figure 2. Binocular UDVA at 3 months postoperative stratified by residual refractive astigmatism in the dominant eye and in the worse eye (the eye of the patient with higher magnitude of residual astigmatism).
A similar trend was observed in patient-reported satisfaction with vision (Figure 3). The percentage of very dissatisfied or dissatisfied patients (rated on a 5-point Lickert scale from very satisfied to very dissatisfied) gradually increased with increasing residual cylinder. Overall, 5.1% of patients reported dissatisfaction if the residual astigmatism in their dominant eye was less than 1.00 D, whereas 9.2% reported dissatisfaction if the residual cylinder was 1.00 D or more (P < .01). Similar percentages in dissatisfaction rates were seen with stratification based on worse eye (4.9% dissatisfaction for astigmatism < 1.00 D vs 8.6% dissatisfaction for astigmatism ≥ 1.00 D, P < .01).
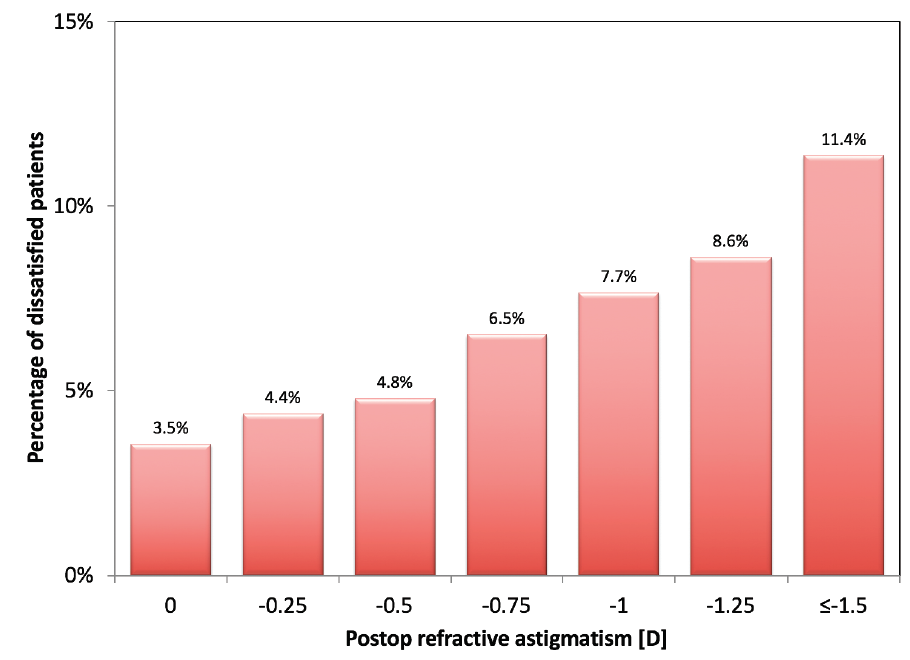
Figure 3. Dissatisfaction with vision (percentage of very dissatisfied and dissatisfied patients) at 3 months postoperative stratified by residual refractive astigmatism in the worse eye.
DISCUSSION
Residual astigmatism is particularly important in refractive surgery, as it can degrade visual acuity and compromise the performance of premium IOLs.5 In the study described here, we demonstrated how even a low level of residual astigmatism can lessen the chance of attaining 20/20 or 20/16 VA and reduce patient satisfaction. Our findings are in line with other studies in which residual astigmatism and/or ametropia were one of the main determinants of patient dissatisfaction;1,2 our study further makes clear that this applies for very low levels of residual cylinder.
Astigmatism is also a common reason for enhancement after multifocal IOL implantation. A previous study found that 75.6% of eyes that required an enhancement had 1.00 D or more of residual refractive cylinder.6
In our large population, we found that every 0.25 D of increased residual astigmatism reduced monocular visual acuity and increased the percentage of dissatisfied patients. Thus, we believe it is worth including low amounts of preoperative astigmatism (≥ 1.00 D) in surgical planning. Nevertheless, precise determination of the magnitude and orientation of corneal astigmatism is of utmost importance,7 and these determinations can often be sources of error.
In recent years, the role of posterior astigmatism and its contribution to total corneal astigmatism has gained recognition, particularly for calculations of toric IOL power.8 Several devices can estimate the magnitude of total corneal astigmatism, although with varying repeatability and precision.9 A widely used biometry device, the IOLMaster 700 (Carl Zeiss Meditec), has incorporated the measurement of posterior astigmatism in its latest swept-source OCT iteration with promising outcomes.9 This allows clinicians to perform customized, all-in-one biometric measurement to effectively and efficiently aid in surgical planning.
Other factors that affect postoperative astigmatism should not be underestimated. Surgeons could improve their outcomes by monitoring and controlling their individual surgically induced astigmatism, allowing them to consistently predict its effect and then incorporate this into surgical planning.4 Further, with the use of toric IOLs, inadequate rotational alignment can limit the efficacy of astigmatic correction, and we should strive to minimize such errors.
CONCLUSION
Many variables can contribute to residual refractive cylinder after cataract surgery. Recognizing that even low levels of postoperative astigmatism can affect visual results and patient satisfaction outcomes after premium IOL implantation is the first and most important step in minimizing its impact.
1. de Vries NE, Webers CA, Touwslager WR, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37(5):859-865.
2. Woodward MA, Randleman JB, Stulting RD. Dissatisfaction after multifocal intraocular lens implantation. J Cataract Refract Surg. 2009;35(6):992-997.
3. Hoffmann PC, Hutz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36(9):1479-1485.
4. Rubenstein JB, Raciti M. Approaches to corneal astigmatism in cataract surgery. Curr Opin Ophthalmol. 2013;24(1):30-34.
5. Hayashi K, Manabe S, Yoshida M, Hayashi H. Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens. J Cataract Refract Surg. 2010;36(8):1323-1329.
6. Gundersen KG, Makari S, Ostenstad S, Potvin R. Retreatments after multifocal intraocular lens implantation: an analysis. Clin Ophthalmol. 2016;10:365-371.
7. Gupta PC, Caty JT. Astigmatism evaluation prior to cataract surgery. Curr Opin Ophthalmol. 2018;29(1):9-13.
8. Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080-2087.
9. LaHood BR, Goggin M. Measurement of posterior corneal astigmatism by the IOLMaster 700. J Refract Surg. 2018;34(5):331-336.




