
Given the choice, I believe most patients would prefer not to wear glasses after cataract surgery. Why, then, have presbyopia-correcting IOLs failed to capture a larger share of the market, despite having been available for more than a decade? Two obstacles—cost and outcomes—regularly seem to limit patient acceptance of these technologies. For some patients, paying out of pocket for a better visual result (in terms of spectacle independence) will never be an option, and for many others the perceived value of investing in their surgery will be determined by what they expect to gain in return. Poor performance in low-light situations, significant and bothersome dysphotopsias, and an incomplete or inadequate range of vision are all factors that have decreased patient satisfaction and limited the value proposition for presbyopia-correcting IOLs in the past.
A CHANGING LANDSCAPE
Into this landscape a new lens has emerged to challenge our thinking of what is possible and what our patients’ experiences can be after cataract surgery. The PanOptix IOL (Alcon, Figure 1) is a trifocal IOL based on quadrifocal technology.1 The design of the lens allows a virtually flat defocus curve,2 meaning that 20/20 VA is possible at distance, near, and intermediate, and with vision throughout the entire range of 20/25 or better.

Figure 1. The PanOptix IOL.
Figures 1 and 2 courtesy of Alcon
As an investigator in the FDA clinical trials of the PanOptix IOL, my personal results with the lens implant showed that patients experienced excellent visual outcomes.3 In the published data from that study, 99.2% of patients said that they would have the same IOL implanted again.4 The design of the lens allows high light transmission, with 88% of available light utilized at a 3-mm pupil size (Figure 2).4 The large diffractive zone of the PanOptix IOL also means that I do not have to limit candidate selection based on pupil size or angle kappa.
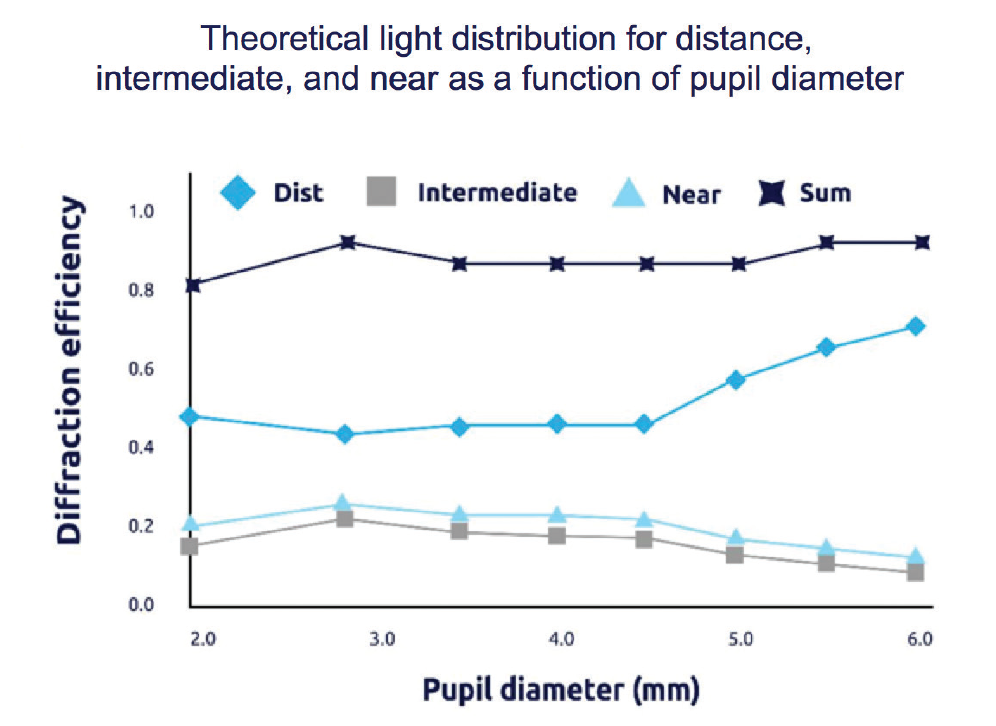
Figure 2. The PanOptix IOL enables 88% of light energy utilization at a 3-mm pupil size4 and allocates 50% of all available light to distance, 25% to intermediate, and 25% to near.
In order to make this technology available to a wider range of patients and surgeons, Alcon released the PanOptix in toric and nontoric versions, as well as in versions that filter or do not filter blue light; all versions include UV filtration. The base platform of the lens is the AcrySof design, which has demonstrated high rotational stability and a low rate of postoperative rotation.5
THE PATIENT EXPERIENCE
The patient experience with PanOptix will be different for most patients than with preceding presbyopia-correcting IOLs. Surgeons should be able to recommend the PanOptix IOL with confidence to a larger group of patients without having to underpromise results.
In my practice, educating patients and discussing their lens implant options has become a simpler process for patients, surgeons, and staff. For patients who desire spectacle independence after cataract surgery, the visual results with the PanOptix lens translate directly into less chair time. I do not have to spend time discussing multiple lens technologies, depending on the patient’s desire to have better near or intermediate vision, or to explain the likely need to combine two different types of implants to achieve a full range of vision.
As always, patients who receive IOLs with diffractive optics should have healthy eyes without significant corneal, macular, or optic nerve pathology. Some patients with an ocular comorbidity may be better suited to implantation with a monofocal or toric monofocal IOL; traditional monovision; an extended depth of focus IOL; a light adjustable IOL; or, in the future, a truly accommodating or pinhole-aperture IOL.
My early experience with the lens since it has become available commercially has demonstrated high patient satisfaction, excellent word-of-mouth referrals, and increasing adoption rates for the technology. I believe this is notable because I already enjoyed a high rate of patient conversion with earlier multifocal IOLs, and I felt that I had achieved the highest possible adoption rates for presbyopia correction after cataract surgery. I have been amazed by the patient-driven demand for the PanOptix in my practice and the corresponding increase in premium IOL surgeries I now perform.
INTO THE OR
Intraoperatively, the PanOptix IOL has been easy to use, which helps to enhance the patient experience. I perform image-guided laser cataract surgery and use intraoperative aberrometry in all of my premium IOL patients; however, excellent results can be achieved with the PanOptix even when more traditional surgery is combined with modern IOL formulas. In the FDA trials, we were allowed to enroll patients with up to 1.00 D of astigmatism in any meridian, but we were not allowed to perform any astigmatism correction, including on-axis, at the time of cataract surgery. Further, only the nontoric version of PanOptix was used in the study.
Even with those study parameters, excellent outcomes and extraordinarily high patient satisfaction were achieved, demonstrating the forgiving nature of the IOL. Small amounts of residual spherical and cylindrical refractive error appear to be well tolerated by most patients with the lens. Part of this can be explained by its very flat defocus curve,2 which demonstrates excellent distance visual acuity with either slight residual myopia or hyperopia. Because of this, surgeons should always target the IOL power closest to plano, rather than erring on the side of slight myopia as we have traditionally done with monofocal IOLs. In the FDA study, we were required to use the Barrett formula to select the PanOptix IOL power that would yield the calculated result closest to plano.
POSTOPERATIVE RESULTS
My patients have experienced excellent immediate satisfaction in their first operated eyes, leading to less hand-holding between eyes and less chair time after surgery. I counsel patients before surgery about the possibility of night dysphotopsias, the need for normal amounts of light for reading without glasses, and the possible need for glasses for some visually demanding activities such as low-light reading. With appropriate and realistic expectations set preoperatively, my patients appear to be satisfied postoperatively.
The full and natural range of vision afforded by the design of the PanOptix provides a positive early and late patient experience. Patients are able to read comfortably at near, and intermediate functions such as computer use are easily accomplished for most patients due to optimized intermediate vision at 60 cm, rather than the 80 cm intermediate distance used in some other trifocal IOLs. Patients report excellent distance vision with the lens without complaints of blur or distortion.
CONCLUSION
The PanOptix IOL has changed the patient experience with premium IOLs in my practice by allowing me to achieve a consistent, reliable result with the highest levels of patient satisfaction. I feel that I can now reliably offer the fullest range of vision to my cataract surgery patients without unacceptable compromises. Patients can enjoy a simplified and easy-to-understand process to help them achieve their goal of spectacle independence.
1. Data on file with Alcon.
2. AcrySof PanOptix Trifocal IOLs [package insert]. Alcon Laboratories. 2019.
3. Fisher BL. Cumulative visual acuity of a novel trifocal IOL. Paper presented at: the 2019 ASCRS/ASOA Annual Meeting; May 3-7, 2019; San Diego, California.
4. AcrySof IQ PanOptix Trifocal IOL [directions for use]. Fort Worth, Texas: Alcon. 2019. https://www.accessdata.fda.gov/cdrh_docs/pdf4/P040020S087D.pdf. Accessed December 13, 2019.
5. Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;125(9):1325-1331.




