Two decades have passed since the debut of a novel IOP-lowering agent for glaucoma management. Netarsudil ophthalmic solution 0.02% (»Rhopressa; Aerie Pharmaceuticals) has ended that dry spell; the topical therapeutic eye drop was approved by the FDA in late 2017 for lowering elevated IOP in patients with open-angle glaucoma or ocular hypertension. Netarsudil represents a new class of compounds—Rho kinase (ROCK) inhibitors—and it has a unique mechanism of action (MOA) that results in increased outflow of aqueous humor through the trabecular meshwork.
“In many ways, Rhopressa is the result of a glaucoma surgeon’s view of what might be an ideal IOP-lowering medication,” said Nathan M. Radcliffe, MD, a glaucoma surgeon specializing in microinvasive glaucoma surgery (MIGS) at New York Eye Surgery Center. “David Epstein, MD, co-founder of Aerie Pharmaceuticals and a glaucoma clinician-scientist pioneer, purposefully set out to develop a drug that could provide the functionality that every glaucoma surgeon sought: to enhance the eye’s natural outflow functionality through the trabecular meshwork. His legacy will be that he found success with this new mechanism—the ROCK inhibitor—that represents the first novel class of IOP-lowering medication to receive FDA approval and reach the glaucoma market in 20 years.”
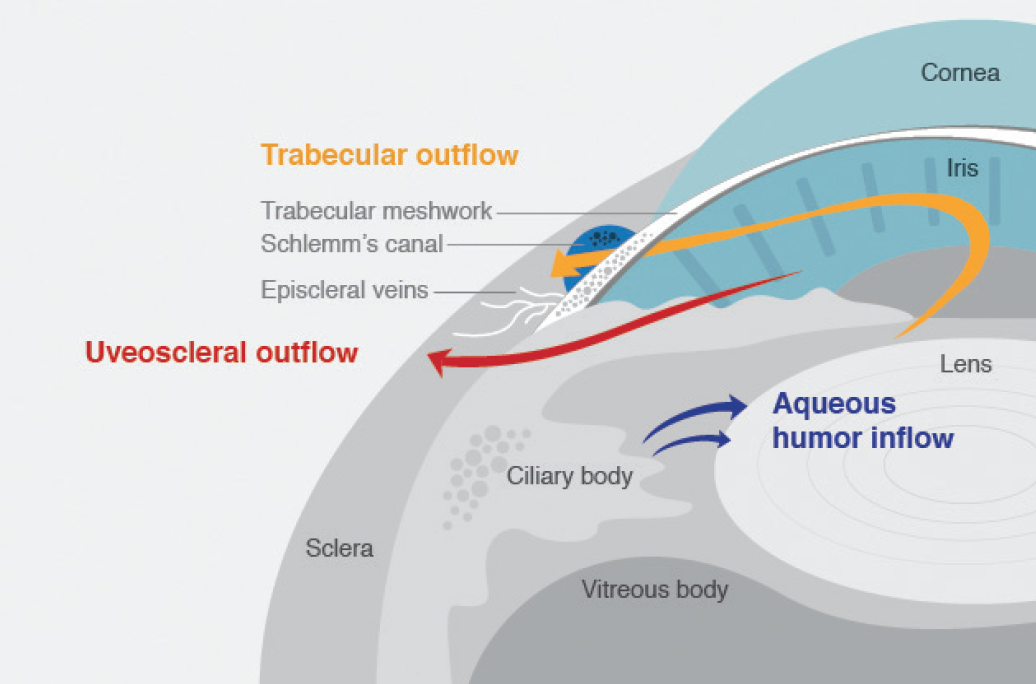
Images courtesy of Aerie Pharmaceuticals
Figure 1. Schematic of the anterior segment highlighting anatomy relevant to IOP homeostasis. Netarsudil ophthalmic solution 0.02%, the first FDA-approved agent in a new treatment class (ROCK inhibitors) for the reduction of IOP, is believed to improve trabecular meshwork outflow.
FDA approval was based on data from the Rocket 1, 2, and 4 randomized, controlled clinical trials, which compared netarsudil once daily in the evening with timolol 0.5% twice daily. In Rocket 2, netarsudil demonstrated consistent IOP lowering across 12 months and at all IOPs tested.1,2 Netarsudil once daily in the evening was comparable in efficacy to timolol 0.5% twice daily in Rocket 1 and 2 clinical trials in patients with IOP under 25 mm Hg and in Rocket 4 in patients with IOP under 30 mm Hg.1-3
Glaucoma specialists interviewed for this article say the once-daily eye drop is a welcomed addition to their therapeutic armamentarium, and its profile suggests that it can be used effectively alone or with other medications to help patients reach and maintain their target pressures. Jason Bacharach, MD, director of research at North Bay Eye Associates in Sonoma, California, and faculty at California Pacific Medical Center, San Francisco, said availability of this new class of therapy should be a source of excitement for everyone who manages glaucoma. “Rho kinase inhibitors work very well as an adjunct to prostaglandins in lowering IOP. In addition to use as a first-line agent in certain cases, their synergistic effect with prostaglandins will bode well for their initial use as an adjunct when IOP is affected by prostaglandins but more reduction is needed,” he said.
Dr. Radcliffe noted the benefits of netarsudil’s potential use as an adjunctive agent. “In an era when the systemic safety profile of other twice-daily, single-agent adjunctive medications has become somewhat complex, Rhopressa will simplify things for eye care practitioners.”
The availability of this new treatment is particularly timely, he said. “Today, glaucoma physicians are looking to restore natural flow through minimally invasive surgical techniques or through pharmacotherapy. Its unique mechanism coupled with its novel medication class will likely make Rhopressa an attractive therapeutic option in 2018.”
Glaucoma specialist Janet B. Serle, MD, pointed out that netarsudil’s benefits include having stable efficacy in eyes with lower and higher baseline IOPs and that its approval expands treatment options for patients (Figure 2). Dr. Serle is a professor of ophthalmology and the glaucoma fellowships director at the Icahn School of Medicine at Mount Sinai Hospital in New York. “Rhopressa, dosed once daily in the evening, has efficacy comparable to the two most commonly prescribed first-line agents, and has mostly mild and tolerable side effects. This profile makes it a first-line option for lowering IOP,” she said.
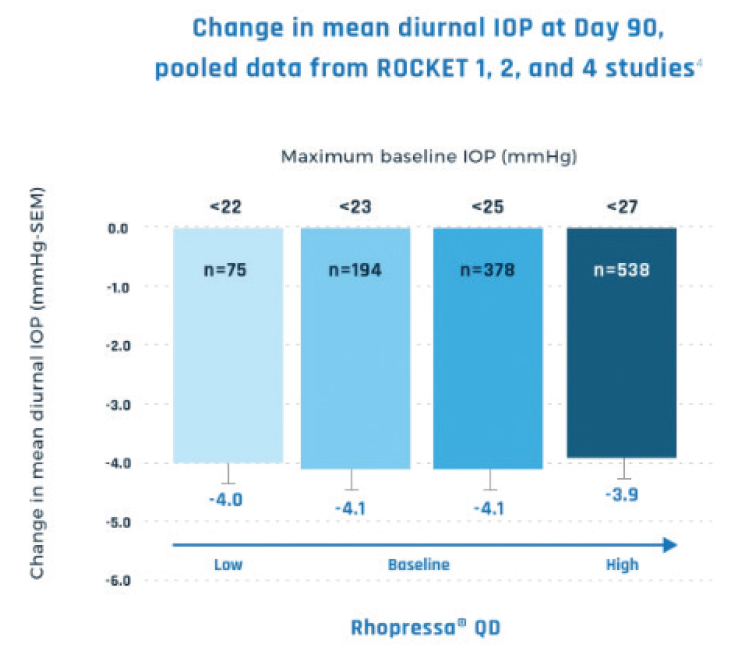
Figure 2. Netarsudil offered consistent IOP reduction, even in patients with a low baseline IOP.
Because netarsudil acts at different receptors than other currently available drugs, Dr. Serle said she expects that it will be additive in efficacy to the other classes of IOP-lowering compounds that act primarily by suppressing aqueous humor formation.
Additionally, the single-dose regimen may have an impact on patient adherence. “The once-daily dosing regimen is expected to enhance compliance and simplify patients’ daily routines,” Dr. Serle said. “Rhopressa will be beneficial for our patients with glaucoma as first-line therapy and as adjunctive therapy. This drug should also be considered for patients who have been intolerant to or have had inadequate efficacy with other medications, patients who are poorly compliant with two- and three-times daily dosing regimens, elderly patients who need additional IOP reduction to avoid surgical intervention, and patients requiring additional IOP reduction to achieve stability of their disease. It is expected that many patients will benefit from the addition of Rhopressa to our treatment armamentarium and that this new drug will assist in reducing the rate of visual disability and progression of glaucoma,” she said.
Constance O. Okeke, MD, MSCE, an assistant professor of ophthalmology at Eastern Virginia Medical School in Norfolk, Virginia, said the availability of a new drug such as netarsudil will significantly affect how she manages patients. “Even though we are in the midst of an explosion of surgical treatments for glaucoma, we still heavily use eye drops. Eye drops will still be utilized for a long time because they have been our go-to for treatment of glaucoma, so this new treatment can have a major impact.”
Dr. Okeke went on to explain, “There are different scenarios that will come up with glaucoma patients, where they may not be amenable to having either laser treatment or surgery first. Some might prefer to try a medication, or there are patients who are on various medications and have problems with tolerability, or there may be situations where we have tried laser treatment—or even surgery—and we just need their IOP a few points lower, but the prospect moving on to a more invasive option does not appeal to the patient. All of these scenarios represent instances where being able to consider a new drug that utilizes a different MOA could be beneficial.”
Beyond that, she adds, it is exciting that netarsudil has a profile that makes it synergistic with other medications. “Whether it is used as a standalone or in conjunction with other treatments, it will play a significant role in glaucoma. Its regimen, simplicity, and tolerability for adherence is huge. Its efficacy, synergy, and unique MOA for usage make me extremely excited about using it.”
Aerie Pharmaceutical’s other main IOP-lowering product, Roclatan (netarsudil 0.02%/latanoprost 0.005%), is about a year behind netarsudil in development. A fixed dose combination of netarsudil and latanoprost, the eye drop achieved its primary efficacy endpoint in two phase 3 registration trials, Mercury 1 and Mercury 2, and also achieved successful 12-month safety and efficacy results in Mercury 1.4 The new drug application (NDA) submission for the combination drug is expected to take place in the second quarter of this year.
According to Dr. Bacharach, netarsudil’s evident synergy with prostaglandin analogues bodes well for netarsudil/latanoprost, which could become the first fixed-dose combination with latanoprost in the United States. Dr. Serle added, “We know from Roclatan studies that Rhopressa and latanoprost are additive. Roclatan lowered IOP better than each of its individual components in a statistically significant fashion in the Mercury 1 and Mercury 2 phase 3 clinical trials,” she explained.4
1. Serle JB, Katz LJ, McLaurin E, Heah T, Ramirez-Davis N, Usner DW, Novack GD, Kopczynski, CK for the ROCKET-1 and ROCKET-2 Study Groups: Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure. Am J Ophthalmol. 2018;186:116-127.
2. Bacharach J, Heah T, Ramirez N, Kopczynski CC, Novack GD. AR-13324 ophthalmic solution 0.02%: topline results of two phase 3 clinical studies in patients with open angle glaucoma and ocular hypertension. Paper resented at: American Glaucoma Society Annual Meeting. March 3-6, 2016; Fort Lauderdale, Florida.
3. Khouri AS, Heah T, Kopczynski CK, Novack GD, and the ROCKET-4 Investigator Group. A double-masked, randomized, parallel study of Netarsudil Ophthalmic Solution, 0.02% QD compared to timolol maleate ophthalmic solution, 0.5% BID in patients with elevated intraocular pressure (ROCKET-4). Paper presented at: the Association for Research in Vision and Ophthalmology Annual Meeting; May 9, 2017, Baltimore.
4. Serle JB, Lewis RA, Kopczynski CC, Heah T. 3-month interim report of a prospective 12-month safety and efficacy study of topical PG324 (fixed combination of netarsudil 0.02% and latanoprost 0.005%) compared to the individual components in subjects with elevated intraocular pressure. Paper presented at: the Association for Research in Vision and Ophthalmology Annual Meeting; May 9, 2017; Baltimore.




