
Early LASIK Patients are Often Best Served by Monofocal IOLs
By Marguerite B. McDonald, MD, FACS
LASIK reached a major milestone in the United States this year: It turned 20. (The first FDA approval for LASIK was granted in 1998.) This turning point does more than signify longevity; it means that a vast population of patients with previous LASIK are either currently ready for cataract surgery or will be in coming years.
Early LASIK treatments were often somewhat decentered, as there was no pupil centration or eye-tracking technology for early excimer lasers (Figure 1). The earliest approved excimer lasers used closing or opening diaphragms to determine laser ablation patterns, rather than flying spots; therefore, the ablations were somewhat rough and often induced unintended higher-order aberrations (HOAs).
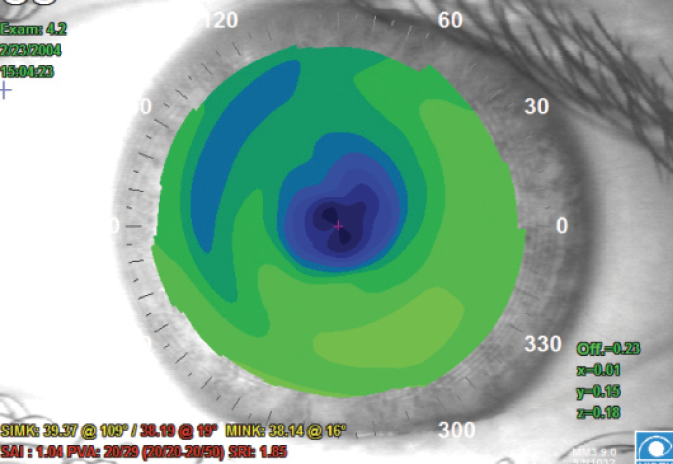
Figure 1. Topography depicting a decentered PRK ablation from a procedure performed in the 1990s. This is representative of the type of patient who should receive a monofocal IOL at the time of cataract surgery.
(Courtesy of Stephen D. Klyce, PhD)
For many patients who had early LASIK procedures and are now facing cataract surgery, therefore a monofocal IOL may be the best choice. Patients who have undergone LASIK more recently have better centered ablations, larger optical zones, and fewer HOAs, so they have a wider range of IOL choices.
High Expectations
Early LASIK patients were, by definition, early adopters, so you can be sure that they will have done their research when they present for a cataract consultation. They will ask for the latest multifocal or extended depth of focus (EDOF) IOL, and it will require some chair time for you to explain your recommendation. It is important to set realistic expectations for these early LASIK patients and explain why you are recommending a monofocal IOL instead of a premium IOL. Once again, more recent LASIK patients may have more IOL choices.
Ocular Surface Disease
In addition to their high expectations, these patients may present with other challenges, such as preexisting ocular surface disease (OSD). It is crucial to get their OSD under control before taking biometry measurements. I follow a detailed treatment algorithm to address OSD, using osmolarity as a guide to my dry eye treatment ladder, as well as slit-lamp examination and meibography to determine the level of blepharitis and appropriate treatment level.
Strategy
My overall strategy for previous LASIK patients is this: I use preoperative »IOLMaster 700 (Carl Zeiss Meditec) measurements, the ASCRS IOL calculator (iolcalc.ascrs.org) for eyes after corneal refractive surgery, and intraoperative aberrometry with the »ORA System with VerifEye+ (Alcon) to determine the appropriate IOL power. I most often recommend a monofocal IOL, especially for patients who had LASIK in its early years because those procedures were frequently associated with unintentional multifocal corneal ablations.
Postoperatively, I prescribe steroid drops eight times a day for 2 days to decrease the chance of late-onset diffuse lamellar keratitis (DLK); then on day 3, I revert to my usual 1 month tapering regimen. To detect DLK, I sometimes see these patients one extra time postoperatively, at 1 and 3 days and 1 week.
CONCLUSION
When cataract patients with previous LASIK are evaluated, especially those who had surgery 15 or more years ago, it is important to obtain topography maps to rule out small, decentered, or irregular ablations. If topography raises any concerns about the ablations being too small, decentered, or irregular, it is always best to use a monofocal IOL. If a multifocal or EDOF IOL is implanted in an early LASIK patient with suboptimal topography maps, this may result in an unhappy patient with significant HOAs and visual disturbances.

Know What You’re Working With
By Robert K. Maloney, MD
Several times a week, I perform cataract surgery on patients who previously had LASIK. When I started doing LASIK in 1991, the average age of these patients was 43. They are now 70—prime age for cataract surgery—and their refractive surgery places them in a unique category that affects my choices with respect to their care.
Most of these patients want good UCVA after cataract surgery. The desire to be less dependent on spectacles is what drove them to have LASIK in the first place. To satisfy the expectations of this patient population, I typically turn to the »Crystalens (Bausch + Lomb) or a toric IOL (see Case Study No. 1). I disagree with those who say that multifocal IOLs work well in these eyes; that has not been my experience.
CASE STUDY NO. 1
These images represent the topography of a 63-year-old man presenting for cataract surgery with previous LASIK. A 2.00 D toric IOL was implanted in his right eye, resulting in a postoperative UCVA of 20/20.
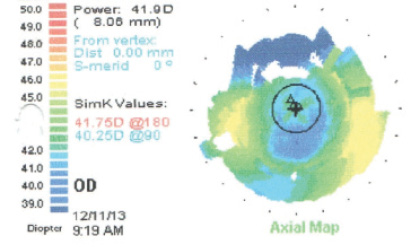
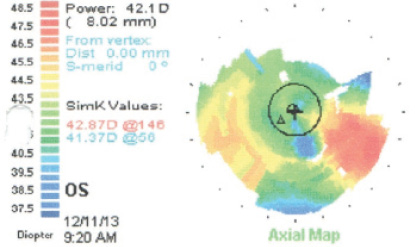
(Courtesy of Robert K. Maloney, MD)
- 63-year-old man
- 2006: LASIK performed elsewhere
- 2015: Complaints of blurry distance vision
- Manifest refraction (MR) OD: -1.50 -2.00 x 93º = 20/25+
- MR OS: -4.75 -4.00 x 82º = 20/30-
- 2+ nuclear sclerotic (NS) cataract OD; 3+ NS cataract OS
- Keratometry readings (Ks): 40.00/42.00@22º
- Ks: 40.50/43.00@151º
- Implant: 2.00 D Toric IOL OD
- Postoperative: UCVA OD 20/20 (MR +0.75 -0.25 x 167º)
Understand the Cause
When we plan cataract surgery for a former LASIK patient, it is important to know two things.
First, is their visual loss caused by the cataract, or it is a result of irregular astigmatism from LASIK? This can be tricky to ascertain because, in an eye with irregular astigmatism, a mild nuclear cataract can cause visual loss disproportionate to the degree of the cataract. Presumably, mild asphericity from the cataract can combine with mild asphericity induced by LASIK to cause this significant visual loss. This supports a more aggressive approach for mild cataracts in patients after LASIK.
On the other hand, removing the cataract but failing to improve the patient’s vision can anger the patient, particularly if he or she has paid for a premium lens. Several approaches may be helpful. Clinical history can tell if the poor vision was of recent onset, which suggests a cataract, or if it is of long standing, which is consistent with irregular astigmatism. Topography can give an idea of how much irregular astigmatism there is. Over-refraction with a gas-permeable lens can be a helpful approach, although it is one that I rarely use.
Complex Calculations
Second, what is the best way to determine the correct IOL power? It is important to remember that the correct IOL power in a patient with prior myopic LASIK will be a higher power than the standard formulas predict; in patients who had prior hyperopic LASIK it will be a lower power than predicted.
To identify the appropriate IOL power, I do preoperative anterior segment OCT (AS-OCT) and topography. I lean toward the median value from several IOL power calculation formulas on the ASCRS IOL calculator. I refrain from using the clinical history formulas, as they are less accurate than those using current data only.
Identifying the best IOL power in post-LASIK eyes with ectasia is even less predictable than it is in the average post-LASIK cataract patient. On the other hand, use of a toric IOL can produce a dramatic improvement in UCVA, even if BCVA fails to improve much.
Laser cataract surgery works well in these eyes. Although the surgical procedure itself is no different, I am cautious about using laser limbal relaxing incisions in these eyes because the preoperative assessment of corneal astigmatism is less reliable than in normal eyes. Unlike with eyes after radial keratotomy (RK), the follow-up schedule for post-LASIK patients is the same as after routine cataract surgery.
AT A GLANCE
- A vast population of patients with previous LASIK are either ready for cataract surgery now or will soon be.
- Calculating the correct IOL power in patients with previous LASIK requires extra attention.
- Ocular surface disease is an added concern in patients with a history of LASIK.
Basement Membrane Dystrophy
Whether in patients who previously had LASIK or in unoperated eyes, the most common cause of poor vision after cataract surgery is unrecognized preoperative basement membrane dystrophy. It is important to do a careful preoperative examination for this condition. If you find central basement membrane dystrophy, the treatment is debridement of a 7-mm zone followed by biometry no sooner than 30 days later.

SAME STRATEGY, MORE DATA
By A. John Kanellopoulos, MD
Over the past few years, we have performed cataract surgery in a large number patients who had previously undergone LASIK, PRK, and even RK. Of these patients, we have had to enhance essentially only one, and most have fallen within ±0.50 D of intended correction. As early LASIK patients reach the age of cataract formation, it is likely that we will continue to encounter more of them in our practice every day, especially because of the excellent results we are achieving. For the most part, we treat these patients like routine cataract patients. We do, however, discuss the fact that their history of having a previous corneal laser procedure makes it more difficult to calculate the appropriate IOL power, and we reassure them that our results to date have been satisfying. In my experience, patients with previous LASIK have an advantage in comparison to those with previous PRK or RK: they have less epithelial remodeling that may affect and change their cornea refractive status after the clear cornea cataract extraction.
The primary topographic sign indicating that a patient has had myopic laser vision correction (LVC) is central flattening of the cornea. This is invariably evident, even many years after myopic LASIK. If there has been previous myopic LVC, an aspheric IOL would be the best choice as these eyes usually have high spherical aberrations. In a case of previous hyperopic LVC, a nonaspheric IOL should be chosen as the previous correction has already transformed the cornea to be highly aspheric. Besides performing topographic and tomographic corneal evaluations, we ensure that, despite the earlier laser procedure, there is good endothelial cell density. We try to judge, for instance, whether the patient had or has subsequently developed coinciding endothelial cell dystrophy or there was damage to the endothelial cells for some other reason (prolonged previous contact lens abuse), and we preemptively discuss with the patient the possibility that he or she may require an additional corneal procedure, such as an endothelial transplant. This would be particularly important in formulating a refractive goal; our preferred endothelial keratoplasty procedure is Descemet stripping automated endothelial keratoplasty, which, in our hands, induces about 2.00 D of hyperopic shift.
DOING A TWO-STEP
If the patient has irregular corneal astigmatism as a result of the previous procedure—for instance, a decentered ablation, or even mild ectasia—then we have to decide on either staying with the existing compromised corneal optics and implanting the IOL, or making it a two-step procedure. The latter entails first performing a customized—in our hands topography-guided—normalization of the cornea (either as a LASIK enhancement or as a PRK on the flap), and then, usually after 3 months, using the now normalized corneal surface (which would ideally be significantly more aspheric), perform more reliable IOL calculation.
In my opinion, the second scenario is better for the long-term visual rehabilitation of these patients, and I strongly encourage them to go this route.
IOL CHOICE
It has been our experience that, despite the fact that these patients are usually highly motivated and already familiar with multifocal IOLs, they are not ideal candidates for this option. If they insist on a premium lens, we suggest the »Tecnis Symfony IOL (Johnson & Johnson Vision), an EDOF lens that has not been associated, in our hands, with problems in these eyes relating probably to low corneal asphericity (usually > +1).
An aspheric lens is an advantage, except in eyes with previous hyperopic LASIK, in which it makes sense to use a spherical IOL to avoid a superaspheric optical system that could probably compromise visual quality.
DATA COLLECTION
In our effort to achieve the best results, we obtain as many data as possible, including whatever is available from the original refractive procedure. Our standard screening includes Placido-disc topography, »Cassini (iOptics) reflection topography, »Pentacam (Oculus Optikgeräte) images, AS-OCT with epithelial mapping (Optovue, »Avanti), »Lenstar (Haag-Streit) and »IOLMaster interferometry measurements, and endothelial cell density. If there is significant dry eye and/or blepharitis, we treat those conditions first to improve the surface for regularity of the keratometry (K) measurements (see Case Study No. 2).
CASE STUDY NO. 2
A 73-year-old man had RK in the 1990s, followed by LASIK 15 years later to correct hyperopic shift. The patient subsequently had cataract surgery that resulted in a slightly myopic outcome. This was corrected with a myopic LASIK enhancement.
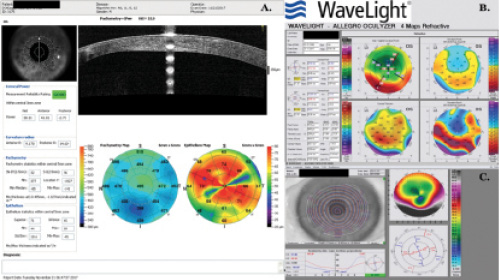
IOL calculation was performed with the ASCRS postrefractive surgery IOL power calculator and with Laservision.gr Research & Clinical Eye Institute’s in-house postrefractive surgery IOL calculation method.
The patient desired ametropic distance correction, so a myopic LASIK enhancement was planned 3 months after cataract surgery.
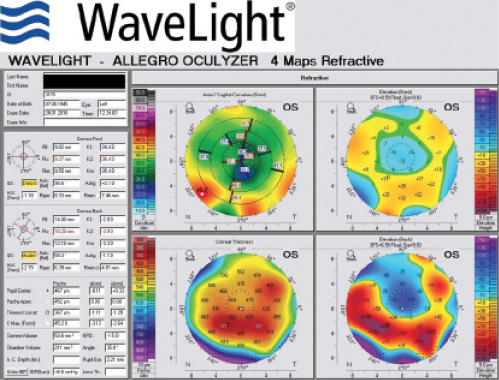
Postoperative corneal OCT illustrates a slightly myopic outcome.
(Courtesy of A. John Kanellopoulos, MD)
SURGICAL STRATEGY
There is no real difference in our cataract surgery strategy for a post-LASIK patient besides the IOL calculation. We are all familiar with the ASCRS IOL calculator for eyes after corneal refractive surgery, including LASIK, PRK, RK, and conductive keratoplasty. It nevertheless sometimes ends up suggesting a wide range of IOL powers. There are currently several helpful formulas that suggest a range of IOLs that could be used, also on the calculator.
As a simple rule of thumb, we also compare the ASCRS calculator data to simply calculating the IOL power using the built-in software on the Lenstar and/or the OA-2000 (Tomey) optical biometer with the current Ks and axial length. If the patient had myopic LASIK, we add 2.00 D to the lens power that is calculated. If the patient had hyperopic LASIK, we subtract 1.50 D from the calculated measurement. Interestingly, the IOL power that we arrive at with this method is typically the same as what is suggested by the ASCRS calculator.
In some cases, in which a significant posterior subcapsular cataract prevents us from achieving proper imaging with interferometry and only immersion ultrasound can be performed, we have used intraoperative aberrometry. As an added measurement, after completing the cataract extraction, we have the patient stand up to be remeasured with the Lenstar and then lie back down to continue the IOL placement with the fresh interferometry measurement.
If an enhancement is needed following IOL implantation, we would not perform a topography-guided enhancement if a toric IOL was used or if the IOL was placed in the sulcus, as this would introduce lenticular astigmatism that cannot be measured by the topography-guided platform. Instead, we perform a wavefront-optimized or even wavefront-guided enhancement using the clinical refraction data.

THE PROMISE OF TOPOGRAPHY
By Alice T. Epitropoulos, MD, FACS
A growing number of patients who have had previous refractive surgery are presenting to our offices for cataract surgery. These patients took steps previously to reduce their dependence on corrective lenses, so their expectations for UCVA after cataract surgery are typically higher than those of the average patient.
Post-LASIK Challenges
A history of refractive surgery makes it challenging for cataract surgeons to reach the refractive target. Measuring corneal power is more difficult in these patients than in previously unoperated eyes, which makes calculating the power of the IOL more complex. For every diopter of correction achieved by laser surgery, the measured corneal power changes by only 0.75 D. Therefore, corneal measurements obtained with standard instruments after myopic LVC are stronger than the true corneal power.
In these situations, I counsel patients that their vision correction outcome may not be as predictable as it would normally be following cataract surgery. They are at higher risk of having a residual refractive error that may require corrective lenses, additional refractive surgery, or even (rarely) an IOL exchange.
Optimize the ocular surface
OSD is common in patients scheduled for cataract surgery,1 especially those who have had previous refractive surgery (Figure 2). A study by Luchs et al found that 59% of patients scheduled for cataract surgery had blepharitis.2 This condition can be associated with an unstable tear film, which can lead to inaccurate K readings, affecting the IOL calculation and subsequent refractive error. Optimizing the health of the tear film (Figure 3) has been shown to result in more accurate preoperative measurements,3 more predictable healing, and overall improved outcomes. I frequently treat this condition using a combination of thermal pulsation, immunomodulators, nutritional supplements, microblepharoexfoliation, and/or lid scrubs containing hypocholorous acid.
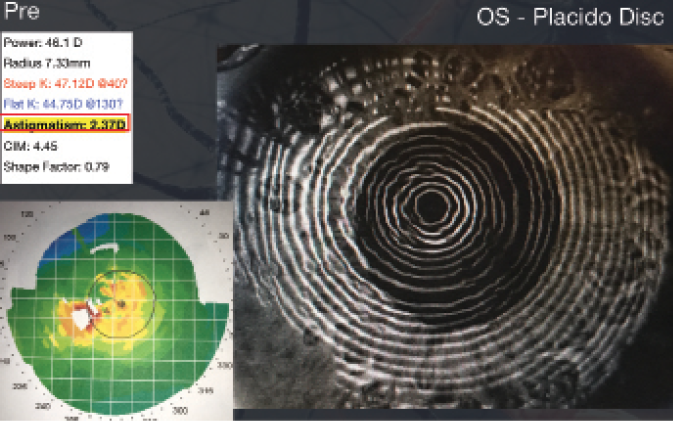
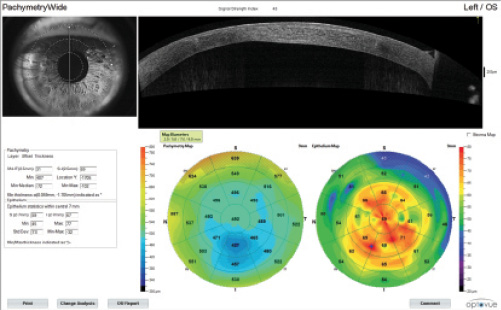
Figure 2. A 68-year-old woman with a history of dry eye disease (DED) and LASIK surgery presented for cataract evaluation. Her DED was causing poor quality topography, irregular mires, and significant pseudocylinder.

Figure 3. After treatment of DED, the patient’s topography showed more regular mires and resolution of pseudocylinder.
(Courtesy of Alice T. Epitropoulos, MD, FACS)
It is beneficial, but not mandatory, to have refractive data from before the LASIK surgery. It is also important to assess the cornea for signs of ectasia or irregular astigmatism preoperatively.
BENEFITS OF TOPOGRAPHY and tomography
Topography and tomography can help to determine whether irregularities in corneal power and shape are contributing to the visual impairment of patients undergoing cataract surgery. This information is especially helpful in patients who have had previous refractive surgery, but these are not considered part of the standard preoperative workup for cataract surgery.4
There are several corneal pathologies, such as irregular corneal astigmatism, OSD, epithelial basement membrane dystrophy, keratoconus, and pellucid marginal degeneration, that cannot be identified with the standard preoperative workup using biometry, slit-lamp examination, and keratometry. These conditions may be better identified with the use of topography or tomography.
Addressing these conditions before proceeding with cataract surgery can improve visual outcomes. Furthermore, corneal topography will show if the patient has undergone previous myopic or hyperopic laser vision correction. Following myopic LASIK, the central cornea is flattened and the midperiphery of the cornea remains relatively unaltered, although at times it may sustain a degree of postoperative scarring due to the flap incision. Topography or tomography will also show whether the ablation was decentered. This information helps to determine if a toric IOL is appropriate. A patient with skewed or irregular astigmatism may not be an ideal candidate for a toric or multifocal IOL.
SURGICAL APPROACH
In patients who have had previous refractive surgery, I usually like to use the ORA System. Wavefront aberrometry provides refractive information including spherical and cylindrical power, along with verifying the axis placement for toric IOLs. This technology also takes into account the contribution of both anterior and posterior corneal astigmatism. All of this information helps to meet those high patient expectations.

Consider Post-LASIK Cataract Patients as Premium IOL Patients
By A. James Khodabakhsh, MD
Every day, I see at least a few patients who previously had LASIK and are now presenting with cataracts. These patients pose a set of challenges different from those of patients with unoperated eyes. The challenges center around performing proper IOL calculations and hitting the desired refractive target. One cannot forget that these patients are still refractive surgery patients, and they are looking for—and demanding—not only that their vision end up at their desired target, but that it also be of good quality.
In my experience, irregular astigmatism and HOAs are the most important things to look for in patients after refractive surgery. This is especially critical among those who have had hyperopic LASIK (Figure 4). I follow a few rules of thumb.
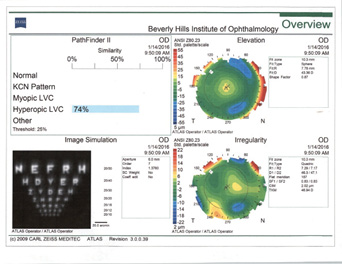
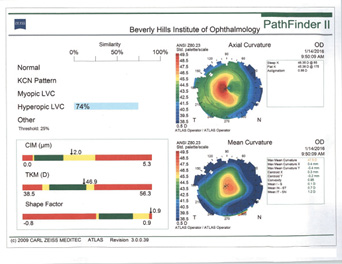
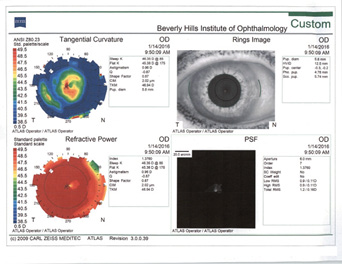
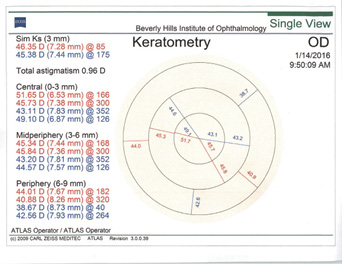
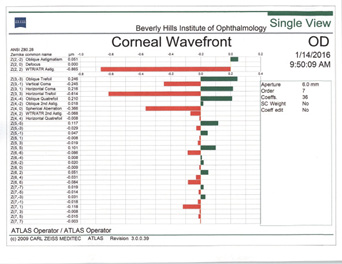
Figure 4. Topographies in a patient presenting for cataract surgery after hyperopic LASIK illustrate central corneal steepening and increased negative spherical aberration along with mild irregular astigmatism. The patient achieved postoperative distance visual acuity of 20/20 after femtosecond-assisted cataract extraction and implantation of an MX60 IOL (Bausch + Lomb).
(Courtesy of A. James Khodabakhsh, MD)
Rule No. 1. I never place a multifocal IOL in an eye with previous hyperopic LASIK (Figure 4). I also never place a monofocal IOL with a negative spherical aberration profile in an eye with previous hyperopic LASIK.
Rule No. 2. If a patient had myopic LASIK (Figure 5) and has smooth topography without any irregular astigmatism, I sometimes consider EDOF or accommodating IOLs.
Rule No. 3. If there is irregular astigmatism and/or significant irregular topography, I avoid diffractive technology IOLs and gravitate toward either a monofocal, toric, or accommodating lens.
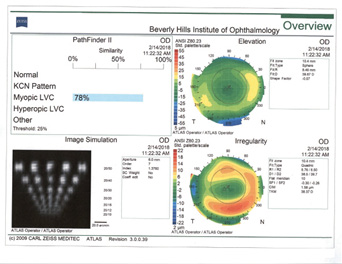
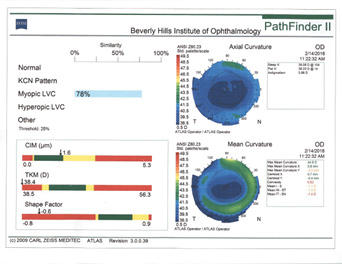
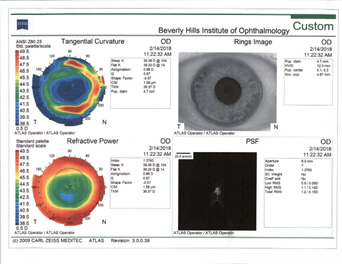
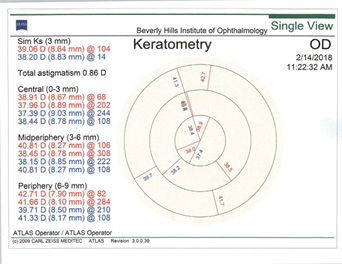
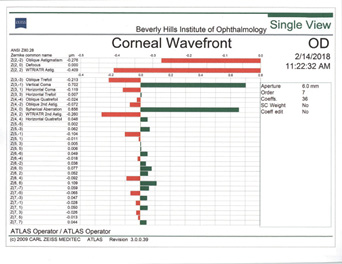
Figure 5. Topographies (top row) and analyses (bottom row) of a patient referred for cataract surgery after myopic LASIK. The patient had central corneal flattening along with positive spherical aberration and regular astigmatism. A Tecnis Symfony Toric IOL was implanted, and the patient achieved excellent postoperative near and distance vision.
(Courtesy of A. James Khodabakhsh, MD)
I think one of the hardest scenarios to deal with among these cataract candidates is the patient with previous hyperopic refractive surgery who insists on being spectacle-free after cataract surgery and does not want monovision correction. These patients are set on getting multifocal IOLs, but they are poor candidates. They pose the greatest challenge because patients who have had hyperopic LASIK already have multifocal corneas. Implanting a multifocal IOL can lead to significant dysphotopsias and patient dissatisfaction, and it takes a lot of chair time to make them understand this.
Cataract Surgery and ORA Intraoperative Aberrometry in a Post-LASIK Case
Diagnostics
My preoperative diagnostic regimen includes obtaining autorefractor K readings, topography, and biometry with either the Lenstar or IOL Master 700. Recent Scheimpflug topographers and AS-OCT can also help with calculations.
I obtain additional calculations using the ASCRS postrefractive surgery IOL power calculator, and I also look closely for dry eye disease (DED) using fluorescein and lissamine green staining. If there is significant dryness and abnormal topography, I start the patient on the proper DED treatment to restore the integrity of the tear film and health of the corneal surface.
Surgical Strategy
My surgical technique is the same in post-LASIK cataract patients as in those with virgin eyes, except that I unequivocally use the ORA System on all postrefractive surgery cataract patients. Because the ratio of the anterior corneal curvature to the unaltered posterior corneal curvature has been changed by the refractive surgery, our formulas cannot provide an exact IOL power in these eyes. Intraoperative aberrometry helps tighten up the results.
My postoperative strategy is the same as for any other premium cataract surgery: Keep the eye quiet to let it heal properly, and correct any visually significant residual refractive error. My follow-up strategy is simple in all premium cataract patients: I see them as often as is necessary to keep them happy. I cannot emphasize enough how imperative it is to treat postrefractive surgery patients who become cataract patients just as you would treat any premium IOL patients.
1. Trattler WB, Majmudar PA, Donnenfeld ED, et al. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
2. Luchs JI, Buznego C, Trattler WB. Asymptomatic or minimally symptomatic blepharitis in patients having cataract surgery. Poster presented at: American Society of Cataract and Refractive Surgery Annual Meeting; May 25-29, 2011; San Diego.
3. Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672-1677.
4. American Academy of Ophthalmology Cataract and Anterior Segment Panel. Cataract in the Adult Eye PPP – 2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. October 2016. Accessed March 8, 2018.




