Lens selection for patients undergoing cataract surgery has historically involved at least a degree of compromise. While monofocal lenses may ensure quality vision at a fixed distance, they do not always provide the same benefit in vision at all ranges of distance. Even within the category of advanced technology lenses, some lens options may offer near vision improvement while sacrificing at least some distance acuity. Indeed, the ability to reliably and predictably provide quality vision across all ranges of distance has long been an unmet need for cataract surgery patients.
Refining the postoperative vision to provide vision that allows patients to resume their regular life activities is one way we may begin to lessen the need for patients to accept compromises in their postoperative vision. This has long been a central tenet of refractive cataract surgery, and the idea has become even more plausible as lens technology has improved. Because of engineering considerations such as the ACTIVEFOCUS Optical Design on the AcrySof IQ ReSTOR IOL (Alcon) platform, surgeons are better equipped to deliver the near visual acuity that patients are after without having to compromise distance visual ability. And, because visual outcomes with this IOL technology are consistent and predictable, the surgeon gains greater flexibility to customize the approach to presbyopia correction.
Uncompromised Distance
Constructed with a seven-step apodized diffractive area, the ACTIVEFOCUS optic efficiently manages light diffusion across vision zones so that more overall light is allocated to distance focal points at any pupil size compared with other IOL designs.1,2 As a result, contrast sensitivity with the AcrySof IQ ReSTOR IOL with ACTIVEFOCUS Optical Design is comparable to a monofocal,1,3,4,5 even for distance viewing tasks (Figure).3 An additional benefit of this design is that it is also designed to reduce the potential for visual disturbances.6
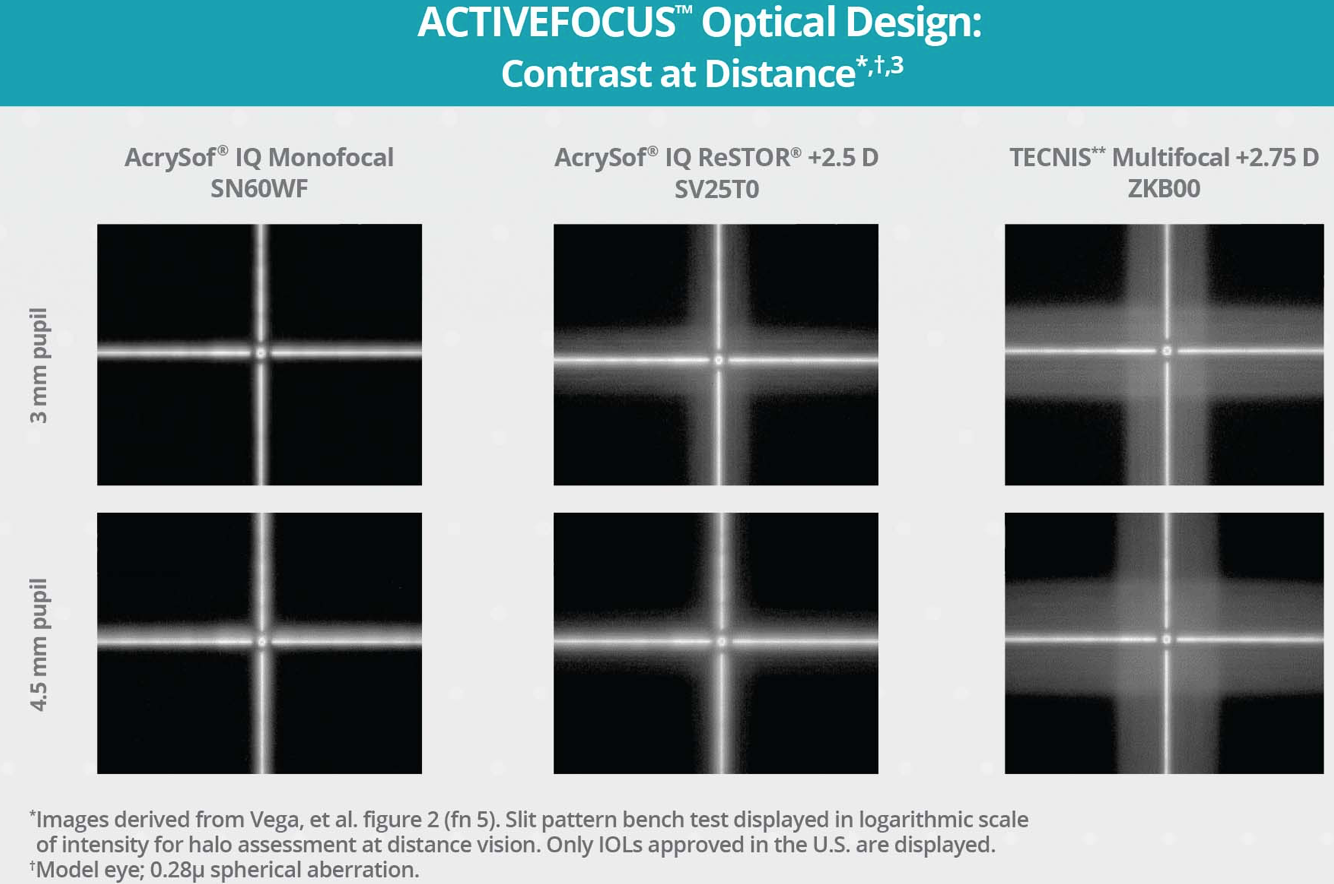
Figure. Contrast sensitivity with the AcrySof IQ ReSTOR IOL with ACTIVEFOCUS Optical Design is comparable to a monofocal,1,3,4 even for distance viewing tasks.
Because the central zone is 100% dedicated to distance vision, the AcrySof IQ ReSTOR IOLs with ACTIVEFOCUS Optical Design offer patients the ability to achieve a full range of vision with uncompromised distance.1,3,5 These factors have added significance when we consider that many cataract patients maintain active lifestyles, including outdoor activities and engaging on social media7—activities that require quality distance visual acuity.
Customizing the Postoperative Vision
One of the advantages of achieving uncompromised distance vision due to the ACTIVEFOCUS Optical Design is that it provides greater flexibility to adjust other parameters to help patients meet their postoperative visual goals. For instance, patients who like to read or paint, use a smartphone, and participate in sporting activities may wish to gain additional near visual ability. In such a patient, it may be plausible to use a ReSTOR +2.5 with ACTIVEFOCUS Optical Design in one eye and a ReSTOR +3.0 in the fellow eye to add up to 2 extra lines of functional near vision without compromising distance vision.7,8
Of note, this strategy of lens selection is fundamentally different than traditional mix-and-match strategies, which include either a blend of lens options or targeting different refractive goals in each eye. Whereas these latter approaches require neuroadaptation and/or a compromise in visual ability in other vision zones, using a blend of ReSTOR +2.5 and +3.0 IOLs with ACTIVEFOCUS Optical Design offers the ability to have quality distance vision in addition to potential gains in near acuity.
Conclusion
The ability to consistently achieve quality distance vision with the ACTIVEFOCUS Optical Design has several obvious benefits and some fortunate consequences as well. Most obviously, quality distance vision allows patients a better chance of leading an active, independent lifestyle.8,9 Yet, the ReSTOR +2.5 with ACTIVEFOCUS optical design achieves near peak performance at 21 inches,3 which is suitable for most near visual tasks. If even greater near acuity is desired, there is an option to use a ReSTOR +3.0 with ACTIVEFOCUS, which achieves near peak performance at 18 inches.10
IOL selection using modern technology platforms does not necessarily have to involve the kinds of visual compromises we have become accustomed to in cataract surgery. The array of options means we are more likely to match patients with an appropriate lens, which, ultimately, enhances the ability to set patients up for success with their postoperative vision.
AcrySof, ACTIVEFOCUS, and ReSTOR are trademarks of Novartis. © Novartis 2018. All other brand/product names are the trademarks of their respective owners.
1. Alcon Data on File (April 11, 2016).
2. Alcon Data on File (Oct 17, 2016).
3. AcrySof IQ ReSTOR +2.5 D IOL Directions for Use.
4. Alcon Data on File (Aug 7, 2013).
5. Vega F, Alba-Bueno F, Millán MS, Varon C, et al. Halo and through-focus performance of four diffractive multifocal intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56(6):3967-3975 (study conducted with corneal model eye with 0.28μ spherical aberration).
6. Alcon Data on File (May 17, 2016).
7. Nuijts RM, Jonker SM, Kaufer RA, et al. Bilateral implantation of +2.5 D multifocal intraocular lens and contralateral implantation of +2.5 D and +3.0 D multifocal intraocular lenses: clinical outcomes. J Cataract Refract Surg. 2016;42(2):194-202.
8. Henderson B, Solomon K, Masket S, et al. A survey of potential and previous cataract-surgery patients: what the ophthalmologist should know. Clin Ophthalmol. 2014;8:1595-1602.
9. Alcon Data on File (2015-2016 Presbyopia-Correcting IOL Physician’s Survey n=90).
10. AcrySof IQ ReSTOR +3.0 D IOL Directions for Use.
AcrySof® IQ ReSTOR® Family of Multifocal IOLs Important Product Information
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician.
INDICATIONS: The AcrySof® IQ ReSTOR® Posterior Chamber Intraocular Multifocal IOLs include AcrySof® IQ ReSTOR® and AcrySof® IQ ReSTOR® Toric and are intended for primary implantation for the visual correction of aphakia secondary to removal of a cataractous lens in adult patients with and without presbyopia, who desire near, intermediate and distance vision with increased spectacle independence. In addition, the AcrySof® IQ ReSTOR® Toric IOL is intended to correct pre-existing astigmatism. The lenses are intended to be placed in the capsular bag.
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling for each IOL. Physicians should target emmetropia, and ensure that IOL centration is achieved. Care should be taken to remove viscoelastic from the eye at the close of surgery.
The ReSTOR Toric IOL should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
Some patients may experience visual disturbances and/or discomfort due to multifocality, especially under dim light conditions. A reduction in contrast sensitivity may occur in low light conditions. Visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. Spectacle independence rates vary; some patients may need glasses when reading small print or looking at small objects.
Posterior capsule opacification (PCO), when present, may develop earlier into clinically significant PCO with multifocal IOLs. Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the AcrySof® IQ ReSTOR® IOLs.
Do not resterilize; do not store over 45° C; use only sterile irrigating solutions such as BSS® or BSS PLUS® Sterile Intraocular Irrigating Solutions.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
© 2018 Novartis 8/18 US-RES-18-E-1850








