
As we retina specialists approach the 10-year mark in our near-ubiquitous use of antivascular endothelial growth factor (anti-VEGF) agents to treat wet age-related macular degeneration (AMD), it is interesting to step back and remember a time when the days of retina specialists were mainly spent performing surgery and when “injection days” or dedicated injection lanes were nonexistent. We have most certainly come a long way from AMD treatments involving photodynamic therapy, laser photocoagulation, and transpupillary thermotherapy—all modalities that focused on destroying leaky vessels (and usually healthy tissue along with them).
We now face new challenges, most of which stem from our desire to give patients the best care without straining the health care system beyond its limits, but is our current standard of care sustainable in the long term?
AT A GLANCE
•Despite evidence of the effectiveness of frequent intravitreal anti-VEGF injections, many patients are not receiving the number of injections necessary to achieve the best visual outcomes.
• The sustained dose of drug emitted into the vitreous with encapsulated cell therapy may minimize local and systemic adverse events that can occur with bolus injections.
• Encapsulated cell therapy is safe and reversible, and it has the potential to ease the treatment burden on all parties involved.
AMD TREATMENT: INJECT OFTEN TO MAINTAIN VISUAL GAINS
We know from the pivotal studies of ranibizumab (Lucentis; Genentech) that monthly injections of this anti-VEGF drug have been shown to halt vision loss and even produce visual gains.1,2 Although these studies were ground breaking in terms of improvement in care for AMD, rigorous and often burdensome treatment regimens, compounded by potential injection risks (eg, endophthalmitis, intraocular inflammation, rhegmatogenous retinal detachment, elevation in IOP, ocular hemorrhage), have led us to look for ways to reduce injection frequency.
Several alternative regimens, including as-needed (PRN) injections and, more recently, treat-and-extend (TAE) protocols, have been evaluated. According to the 2015 Global Trends in Retina survey of 586 US retina specialists, more than 90% of respondents utilize alternative treatment regimens or a combination of approaches. Specifically, 12% treat PRN, 66% follow TAE, and 19% use a combination of these approaches.3
PRN treatment schemes can work, but they require monthly patient monitoring and strict re-treatment guidelines to avoid declines in vision. TAE regimens have also been proven to reduce cost and treatment burden with increases in visual acuity; however, this approach still requires close and frequent monitoring and relies heavily on trial and error.
The HORIZON, CATT, and SEVEN-UP trials suggested that the most efficacious treatment strategy for sustained gains in visual acuity consists of at least seven to eight intravitreal injections of anti-VEGF medication during the first year and at least five to six injections during the second year, coupled with frequent follow-up examinations with optical coherence tomography.4-6
ARE PATIENTS UNDERTREATED?
Despite the overwhelming evidence of the effectiveness of frequent treatments, a large survey of Medicare beneficiaries showed that many patients are not receiving the number of injections required to achieve the best visual outcomes.7 The study reviewed claims of 459,237 Medicare beneficiaries from between 2006 and 2010 and showed that the frequency of anti-VEGF injections was lower than that recommended in clinical studies. The study also revealed high discontinuation rates (71% within 24 months). In the first year after initial treatment, 59% of treated eyes received one to four injections, and 22% received seven or more injections, with an overall mean of 4.3 injections.
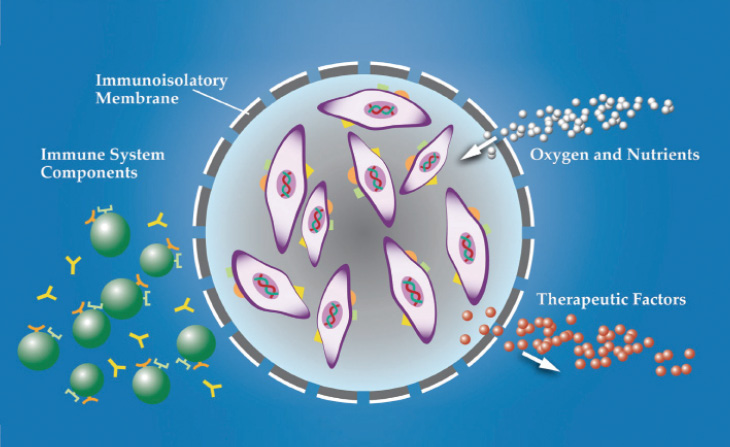
Figure 1. Cross-section of the encapsulated cell therapy (ECT) implant.
Another claims database analysis of more than 19,000 newly diagnosed neovascular AMD patients from between 2006 and 2011 revealed that the mean annual number of ranibizumab or bevacizumab (Avastin; Genentech) injections was between 4.6 and 6.9.8 Again, this is markedly lower than the monthly frequency that provided excellent visual outcomes in controlled clinical trials. This apparent “undertreatment” and “underevaluation” in the real-world setting was corroborated in yet another independent claims analysis of more than 10,000 newly diagnosed AMD patients,9 authored by Szilárd Kiss, MD.
Dr. Kiss, who is director of clinical research and an associate professor of ophthalmology at Weill Cornell Medical College, New York-Presbyterian Hospital, commented, “Data from recent studies by our group and other groups suggest that patients treated with anti-VEGF therapy are monitored less frequently and receive significantly fewer injections in clinical practice compared with patients in major clinical trials. Moreover, as our group and others have shown, these less frequent dosing and follow-up strategies, in turn, lead to inferior visual acuity results in the real-world setting. As a result, an intense area of AMD research has focused on sustained delivery of anti-VEGF agents, both to reduce treatment burden and to perhaps improve visual outcome.”
Because these studies evaluated only claims (not treatment patterns), the association between observed frequency of injections and patient monitoring and clinical outcomes, such as visual acuity, were not assessed. Nevertheless, it has become common knowledge that the number of injections given often correlates with the number of letters gained. Moreover, as injections drop off, maintenance of these gains becomes an issue. In fact, recent studies of visual outcomes in the real-world setting of treatment of newly diagnosed wet AMD patients are beginning to show that the visual acuity gains are far less than those seen in large prospective clinical trials. In an analysis of nearly 340 eyes with newly diagnosed AMD between 2007 and 2012, the mean number of letters gained was 5.3 over the first 12 months, with a mean of 5.5 injections.10 A separate single-center real-world analysis showed even fewer letters (1.9) gained after the first year and a loss of 2.4 letters over 5 years of treatment with ranibizumab in newly diagnosed patients with wet AMD.11 This leads us to ask, “Is it possible to maximize visual outcomes and minimize treatment burden in the long term for our wet AMD patients?”
A NEW TREATMENT PARADIGM
As we attempt to find ways to reduce the number of injections while continuing to provide optimal visual benefits for our patients, it may be time to look for different ways to deliver AMD therapy. A treatment that delivers continuous, long-term therapy to the posterior segment may be advantageous for patients, physicians, and caregivers. An option that could maintain vision over the long term and minimize systemic risk while being more cost-effective and less burdensome could potentially lead to a significant paradigm shift in AMD treatment. Several companies have been working to achieve this goal.
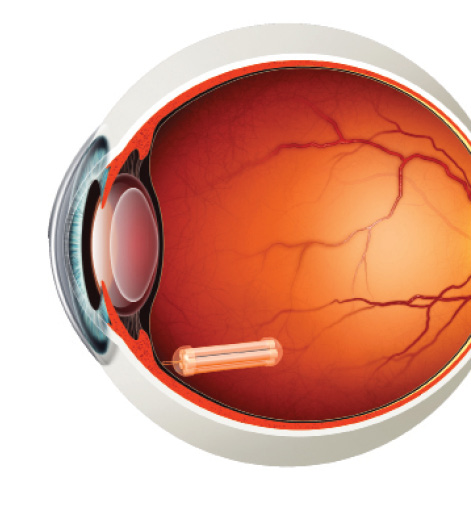
Figure 2. ECT sutured to scleral wall.
Encapsulated Cell Therapy
ECT (Neurotech Pharmaceuticals) is one treatment approach that has a long track record of delivery to the back of the eye. The ECT platform utilizes a proprietary, immortalized, nontumorigenic human retinal pigment epithelial cell line that has been genetically engineered to produce a wide array of therapeutic proteins, including cytokines, monoclonal antibodies, FAB fragments, fusion proteins, and peptides. The cells are housed in individual cartridges, each surrounded by a semipermeable membrane that enables the outward passage of drug and the inward diffusion of oxygen and nutrients without eliciting an inflammatory response (Figure 1). The implant is inserted through a small scleral incision and sutured to the scleral wall (Figure 2) using a titanium clip. It can be easily removed, if necessary.
The ECT platform has many potential advantages over other long-term delivery therapies. It can support the continuous production of therapeutic proteins into the vitreous, singly or in combination with another drug or drugs. ECT has been shown to consistently deliver proteins for at least 2 years to treat a broad range of retinal diseases, although more recent explant data have suggested sustained delivery persists for at least 5 years (data on file with Nuerotech Pharmaceuticals, 2015).In addition, the relatively low sustained dose of drug emitted into the vitreous with an ECT implant may minimize local and systemic adverse events that can occur from bolus injections.
NT-501 ECT
The ECT platform has been used to produce NT-501 ECT (Neurotech Pharmaceuticals), which consists of encapsulated human cells genetically modified to secrete therapeutic doses of ciliary neurotrophic factor into the back of the eye for treatment of several retinal degenerative diseases. Early pilot data in patients with glaucoma suggest an improvement in visual field index and corresponding macular and nerve fiber layer volume as early as 1 month and lasting for at least 18 months.12 To date, NT-501 ECT has been generally safe, with approximately 1,000 person-years of cumulative exposure (data on file with Neurotech Pharmaceuticals, 2015)
NT-503 ECT
Neurotech’s lead ECT product candidate is NT-503 ECT, a soluble VEGF receptor (sVEGFR) fusion protein for the treatment of wet AMD and other neovascular diseases of the retina. In vitro, NT-503 ECT-produced sVEGFR protein binds with picomolar affinity to human VEGF-A, similar to the reported values for aflibercept (Eylea, Regeneron) and with approximately 700 times greater affinity than ranibizumab.
An ongoing dose-escalation study of NT-503 ECT is evaluating three doses and two ECT configurations. Preliminary results in the highest-dose group have shown a clinically meaningful dose response in visual acuity and reductions in macular thickening for at least 20 months, along with a lower injection rate frequency than was seen in the other treatment groups. Additionally, it appears to be safe and well tolerated.
A phase 2 randomized, active-controlled, masked multisite study was recently initiated to evaluate the safety and efficacy of NT-503 ECT compared with intravitreal aflibercept injections. Approximately 150 patients who have demonstrated a good clinical response to at least three anti-VEGF injections will be enrolled. This study will compare maintenance of vision in patients randomized to receive a single NT-503 ECT implant or aflibercept injection every 8 weeks. Efficacy will be evaluated using a combination of endpoints, including change in visual acuity, change in retinal thickness, rate of treatment failures, and rate of rescue medication. A primary analysis will be conducted at 1 year, and patients will be observed for 2 years. Results are expected in early 2017. This represents a shift in how we have traditionally run clinical trials. Most trials are conducted in treatment-naïve patients, and the main outcome is an improvement in vision. Now that we have entered an era in which most patients are not treatment naïve and often require a lifetime of treatment, we must test the durability and longevity of a treatment in maintaining vision over the long term. This is where ECT may play a role.
ECT: THE FUTURE OF LONG-TERM RETINAL TREATMENT?
ECT is a versatile drug delivery platform capable of producing a wide range of therapeutic proteins at constant levels for multiple years. Not only does it have the ability to treat long term with single and combination therapies for at least 2 years, but it is safe and reversible. It may also ease the treatment burden on patients, caregivers, the health care system, and our offices. ECT could very well be the next-generation treatment platform for chronic ocular diseases.
Recent data suggesting improvements in visual field and corresponding improvements in macular and nerve fiber layer volume with NT-501 ECT (ciliary neurotrophic factor) in glaucoma are intriguing. NT-503 ECT (sVEGFR) has been well tolerated and achieved clinically meaningful improvements in visual acuity and reductions in macular thickening for at least 20 months following surgical implantation in patients with wet AMD. NT-503 ECT is expected to have efficacy at least as comparable to existing anti-VEGF therapies, and it will be compared with aflibercept in an upcoming randomized, double-masked, controlled phase 2 trial. This is an exciting time for retina specialists as we determine the best way to treat our patients adequately in the long term. I look forward to the future of ECT.
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
2. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444.
3. Rezaei KA, Stone TW. 2015 ASRS Global Trends in Retina Survey. Paper presented at: American Society of Retina Specialists Annual Meeting; July 11-14, 2015. Vienna, Austria.
4. Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6)1175-1183.
5. CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908.
6. Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292-2299.
7. Lad EM, Hammill BG, Qualls LG, et al. Anti-VEGF treatment patterns for neovascular age-related macular degeneration among medicare beneficiaries. Am J Ophthalmol. 2014;158(3):537-543.
8. Holekamp NM, Liu Y, Yeh W, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol. 2014;157(4):825-833.
9. Kiss S, Liu Y, Brown J, et al. Clinical monitoring of patients with age-related macular degeneration treated with intravitreal bevacizumab or ranibizumab. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):285-291.
10. Almony A. Clinical utilization of anti-VEGF therapy for neovascular age-related macular degeneration – analysis of electronic medical records from a large integrated US health system database. Paper presented at: The Retina Society Annual Meeting; October 7-11, 2015; Paris, France.
11. Zhu M, Chew JK, Broadhead GK, et al. Intravitreal ranibizumab for neovascular age-related macular degeneration in clinical practice: five-year treatment outcomes. Graefes Arch Clin Exp Ophthalmol. 2015;253(8):1217-1225.
13. Goldberg JL. Is neuroregeneration a viable treatment for glaucoma? Paper presented at: American Glaucoma Society Annual Meeting and North American Neuro-Ophthalmology Society; February 21-26, 2015; Coronado, CA.
Allen C. Ho, MD
• attending surgeon and the director of retina research at Wills Eye Hospital in Philadelphia
• professor of ophthalmology at Sidney Kimmel College of Medicine at Thomas Jefferson University, Philadelphia
• chief medical editor of Retina Today
• acho@att.net
• financial disclosure: consultant to Neurotech


