


Treatment of sight-threatening diseases, particularly those of the retina and choroid, poses a familiar challenge. The drug or therapeutic intervention must reach the targeted ocular tissue at the right concentration or at the appropriate frequency to produce the optimal outcome while at the same time minimizing the interaction of the drug with unintended targets. The overall goal is to optimize efficacy at the intended site of action and to reduce the side effects and complications of the drug. This balance is important, even in the local administration of drugs to the eye through topical drops, intravitreal injection, or implantation.
AT A GLANCE
• Administering a drug through the suprachoroidal space results in an apparent compartmentalization of the drug to the posterior segment of the eye without unduly exposing the vitreous and anterior segment.
• One potential advantage of the suprachoroidal delivery of corticosteroids over other intra- and periocular corticosteroid delivery methods is that it may allow lower doses or less frequent dosing than current local treatments, with potentially better efficacy and fewer adverse events.
• Suprachoroidal administration of triamcinolone acetonide has shown promising results in preclinical and early clinical studies.
The unique anatomy of the eye also poses a challenge, in that the target tissues for the majority of sight-threatening conditions are located posteriorly. This requires drugs to traverse the anterior chamber or other outer structures of the eye in order to reach the targeted tissues following local administration. The dilemma in the case of corticosteroid use is how to deliver these drugs to the posterior of the eye without adversely affecting anterior tissues. Administering drugs through the suprachoroidal space of the eye provides a potential solution to this challenge.
SUPRACHOROIDAL DRUG DELIVERY
The suprachoroidal space is an approximately 30-µm thick layer forming a transition zone between the choroid and sclera. It is composed of closely packed layers of long pigmented processes derived from each tissue, and it appears to provide a pathway for uveoscleral outflow.1 Fluid injected into the suprachoroidal space spreads around the globe on top of the choroid, distributing through the choroid and retina. Should a drug be injected, the solution or suspension spreads in a similar manner across the choroid. In contrast, when the same drug is injected into the vitreous, the drug diffuses across all parts of the eye. The suprachoroidal space appears to provide a compartment through which drugs can flow, reducing exposure of the vitreous and anterior segment to the injected entity. Furthermore, providing a more targeted approach to accessing retinal and choroidal tissues results in a higher local concentration of drug in the posterior tissues and may allow lower doses of a drug to be administered or less frequent dosing.
The advantage of the suprachoroidal space as a potential route for drug administration has only undergone limited exploration in the treatment of ophthalmic diseases. For example, drug administration through the suprachoroidal space as a therapy for retinal disease has been studied using a surgical procedure employing the placement of long cannulas for administering the drug.2 However, for extended or long-term therapy requiring multiple treatments, accessing the suprachoroidal space by this method or similar surgical methods is impractical. Furthermore, it is difficult to justify the risk of surgical intervention when no obvious advantage over other local therapies has been demonstrated.
Realizing the therapeutic potential of the suprachoroidal space requires an approach that allows drugs to be introduced into the space in a minimally invasive yet consistent and efficient manner. Therapeutic advantages must be demonstrated through a systematic set of preclinical and clinical studies.
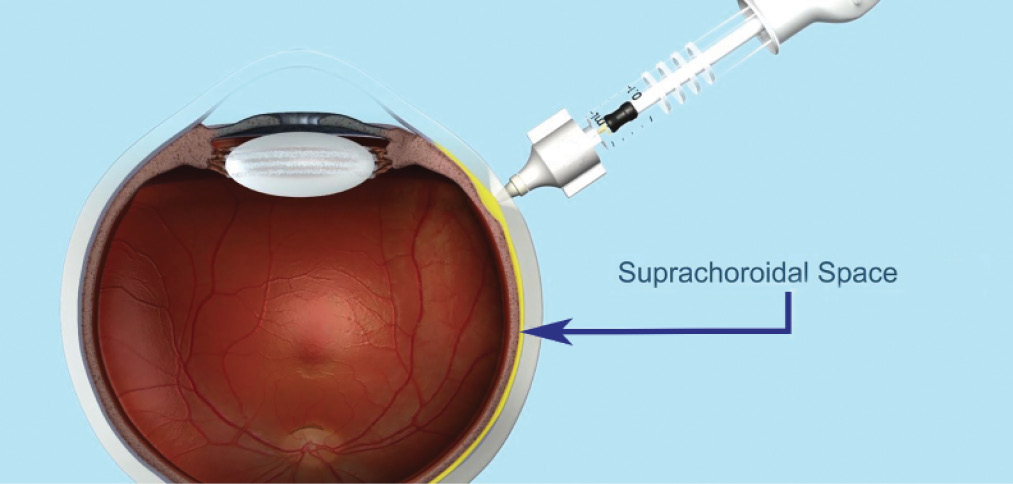
Figure. Administration of drug to the eye via the suprachoroidal space using a proprietary microinjector. Data from in vivo animal models and ex vivo eyes show the injected drug spreading around the eye after suprachoroidal injection.
REVIEW OF RELEVANT STUDIES
Preclinical Studies
In preclinical studies, triamcinolone acetonide (TA) administered through the suprachoroidal space using a proprietary microinjector (Clearside Biomedical; Figure) has shown promising potential for efficacy. In animal models, improved anti-inflammatory activity was seen with suprachoroidal administration of TA at a lower dose compared with TA given by intravitreal injection or maintenance dose prednisone given orally.3-5
Studies measuring the distribution of drug in a rabbit model found higher concentrations of drug in relevant tissues (choroid and retina) and lower concentrations in nontarget areas of the eye (lens, aqueous humor) with suprachoroidal administration than with intravitreal administration.6 Preclinical efficacy studies in a porcine model of acute uveitis found that a 0.2-mg dose of TA injected suprachoroidally was more effective than a 0.2-mg dose of TA administered intravitreally and as effective as a 2-mg intravitreal dose.3 These data demonstrated that a 10-fold lower dose of TA administered to the suprachoroidal space was sufficient to provide efficacy.
In a separate efficacy experiment using the same model, TA administered in the suprachoroidal space was as effective as high-dose daily oral corticosteroid treatment, one of the most commonly used methods of controlling inflammation associated with noninfectious uveitis.4 Injection of TA into the suprachoroidal space resulted in a significantly more rapid onset of anti-inflammatory activity (day 1) than oral prednisone dosing (day 3) and produced efficacy equivalent to high-dose oral prednisone on day 3 and superior to low-dose prednisone, often used in maintenance therapy, on all study days.4
In pharmacokinetic studies in rabbits, distribution of the drug over a 90-day period following a single suprachoroidal injection showed that TA was 12 times more available in the choroid and outer retina compared with distribution following intravitreal administration. In addition, although equal amounts of drug were found in the neural retina, minimal levels of drug were found in the aqueous humor and in the iris and ciliary body with suprachoroidal administration compared with intravitreal injection of TA.6
Single- and repeat-dose toxicology studies in rabbits suggested that TA administered in the suprachoroidal space was tolerable and safe for a 13-week evaluation period following each administration, while systemic drug levels were low to undetectable.7,8
Clinical Trials
On the basis of this encouraging preliminary work, Clearside Biomedical initiated a clinical study to evaluate suprachoroidal administration of TA for the treatment of noninfectious uveitis. Results from a phase 1/2 study in patients with noninfectious uveitis showed improvement in BCVA ranging between 1 and 5 lines (or up to 25 letters). At week 12, the average improvement in BCVA exceeded 2 lines, and at week 26, the average was close to 3 lines. No patient in the trial experienced any meaningful increase in IOP at any time point following suprachoroidal space administration, and none was required to use IOP-lowering medications in the trial (data on file with, Clearside Biomedical, 2015). These findings are encouraging in that they suggest that the adverse effects commonly associated with conventional ophthalmic use of corticosteroids (eg, increases in IOP and lens opacification) may be minimized when TA is administered through the suprachoroidal space.
Two clinical trials are currently enrolling patients to confirm the clinical potential of a proprietary formulation of triamcinolone (CLS-TA; Clearside Biomedical) as monotherapy and in combination with other agents for the treatment of eye diseases. One trial is a phase 2, randomized, masked, internally controlled, multicenter study to assess the safety and efficacy of two doses (4 mg or 0.8 mg) of CLS-TA administered to the suprachoroidal space using the microinjector for the treatment of patients with macular edema associated with noninfectious uveitis. The other is a phase 2, randomized, masked, internally controlled multicenter study to assess the safety and efficacy of suprachoroidally administered CLS-TA in combination with intravitreally injected aflibercept (Eylea; Regeneron) in patients with retinal vein occlusion. Results from these studies, expected this year, will provide ophthalmologists with the most robust and systematic evidence to date of the clinical utility of pharmacologic therapy through the suprachoroidal space for treatment of retinal diseases.
CONCLUSION
In theory, and eventually in practice, the ability to treat retinal diseases by utilizing the suprachoroidal space to administer drugs to the choroid and retina may result in lower doses or less frequent dosing than conventional treatments, with equal or improved efficacy and fewer adverse effects. In the future, therapies that involve the suprachoroidal space may take a prominent place in the armamentarium, providing a new opportunity to improve patients’ care and clinical outcomes across a spectrum of retinal diseases.
1. Hogan MJ, Alvarado JA, Weddell JE. Choroid. In: Histology of the Human Eye. Philadelphia, PA: W.B. Saunders Company; 1971.
2. Tetz M, Rizzo S, Augustin AJ. Safety of submacular suprachoroidal drug administration via a microcatheter: retrospective analysis of European treatment results. Ophthalmologica. 2012; 227(4):183-189.
3. Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54(4):2483-2492.
4. Noronha G, Blackwell K, Gilger BC, et al. Evaluation of suprachoroidal CLS-TA and oral prednisone in a porcine model of uveitis. Poster presented at: Association for Research in Vision and Ophthalmology Annual Meeting. May 3-7, 2015; Denver, CO.
5. Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492-513.
6. Edelhauser H, Patel S, Meschter C, et al. Suprachoroidal microinjection delivers triamcinolone acetonide to therapeutically relevant posterior ocular structures and limits exposure in the anterior segment. Poster presented at: Association for Research in Vision and Ophthalmology Annual Meeting. May 5-9, 2013; Seattle, WA.
7. Verhoeven RS, Patel SR, Viaud-Quentric K, et al. Suprachoroidal microinjection of triamcinolone acetonide is well tolerated in the albino rabbit. Poster presented at: Association for Research in Vision and Ophthalmology Annual Meeting. May 5-9, 2013; Seattle, WA.
8. Noronha G, Edelhauser HF, Patel SR, et al. Safety and toxicokinetics of suprachoroidal space injections of triamcinolone acetonide suspension in albino rabbits. Poster presented at: Association for Research in Vision and Ophthalmology Annual Meeting. May 4-8, 2014; Orlando, FL.
Sara V. Branson, BS
• medical student at Wake Forest University School of Medicine in Winston-Salem, North Carolina
• financial interest: none acknowledged
Claudia G. Hooten, MD
• medical student at Wake Forest University School of Medicine in Winston-Salem, North Carolina
• chooten@wakehealth.edu
• financial interest: none acknowledged
Shree K. Kurup, MD
• director of research and an associate professor of ophthalmology at Wake Forest University Eye Center in Winston-Salem, North Carolina
• skurup@wakehealth.edu
• financial disclosure: consultant for and/or on the advisory board/ steering committee of Abbott, AbbVie, Allergan, Clearside Biomedical, Regeneron, and Xoma


